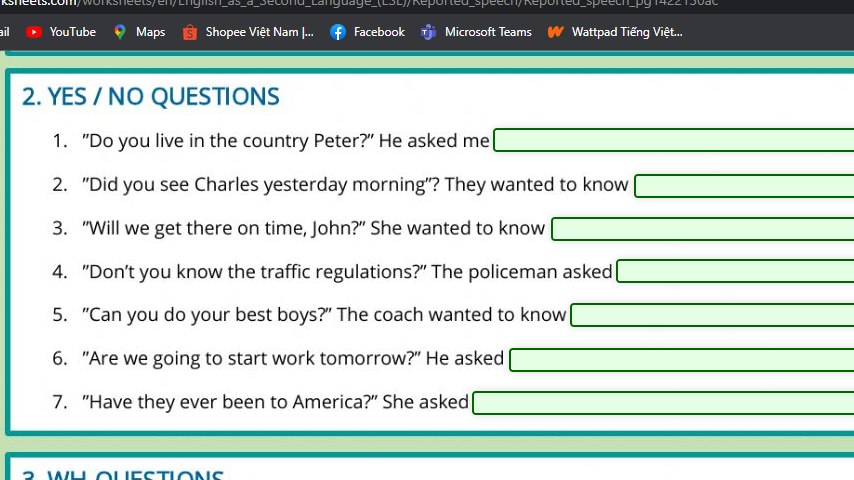
\(1.\)
\(C\%_{dd_{Cu}}=\dfrac{m_{Cu}}{m_{dd_{Cu}}}.100\%\)
\(\Leftrightarrow m_{Cu}=\dfrac{m_{ddCu}.C\%_{ddCu}}{100\%}\)
\(\Leftrightarrow m_{Cu}=\dfrac{170.8\%}{100\%}\)
\(\Rightarrow m_{Cu}=13,6g\)
\(1.\)
tính \(m_{AgNO_3}\) của em đúng á chị thêm thui
PTHH: Cu + 2AgNO3 \(\rightarrow\) Cu(NO3)2 + 2Ag
TL: 1 2 1 2
mol: 0,04 \(\leftarrow\) 0,08
\(m_{Cu}=n.M=0,04.64=2,56g\)
\(2.\)
\(C\%_{ddBa\left(OH\right)_2}=\dfrac{m_{Ba\left(OH\right)_2}}{m_{ddBa\left(OH\right)_2}}.100\%\)
\(\Leftrightarrow m_{Ba\left(OH\right)_2}=\dfrac{m_{Ba\left(OH\right)_2}.C\%_{ddBa\left(OH\right)_2}}{100\%}\)
\(\Leftrightarrow m_{Ba\left(OH\right)_2}=\dfrac{50.12\%}{100\%}\)
\(\Rightarrow m_{Ba\left(OH\right)_2}=6g\)
\(n_{Ba\left(OH\right)_2}=\dfrac{m}{M}=\dfrac{6}{171}\approx0,04mol\)
PTHH: CO2 + Ba(OH)2 \(\rightarrow\) BaCO3 + H2O
TL: 1 1 1 1
mol: 0,04 \(\leftarrow\)0,04
\(V_{CO_2}=n.22,4=0,04.22,4=0,896l\)
\(3.\)
\(n_{Mg}=\dfrac{m}{M}=\dfrac{24}{24}=1mol\)
PTHH: H2S + Mg \(\rightarrow\) MgS + H2
TL: 1 1 1 1
mol: 1 \(\leftarrow\) 1
\(m_{H_2S}=n.M=1.34=34g\)
\(C\%_{ddH_2S}=\dfrac{m_{H_2S}}{m_{ddH_2S}}.100\%=\dfrac{34}{200}.100\%=17\%\)

 làm hộ hóa với ạ dạng công thức tính C% nhất là phần phương trình hóa học ạ
làm hộ hóa với ạ dạng công thức tính C% nhất là phần phương trình hóa học ạ