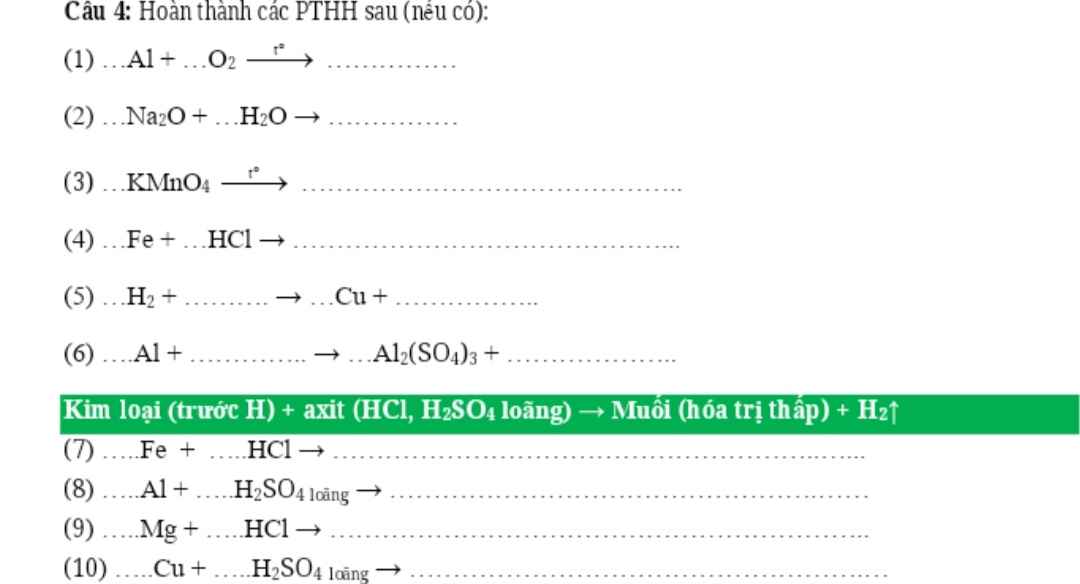
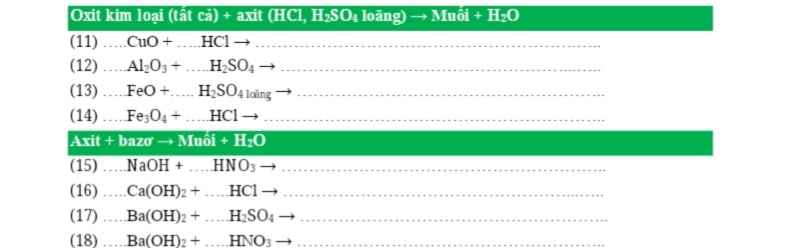
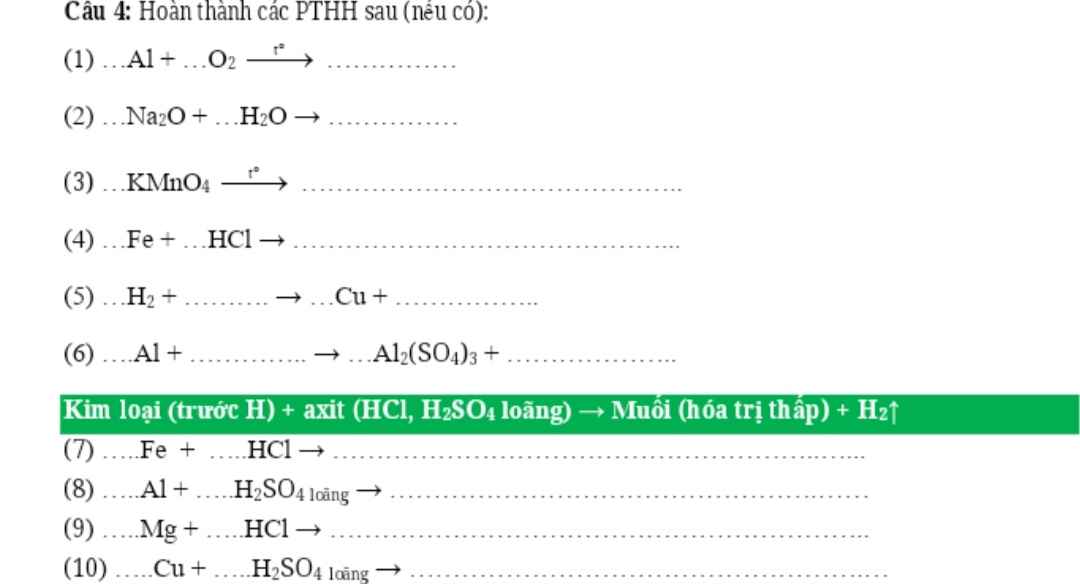
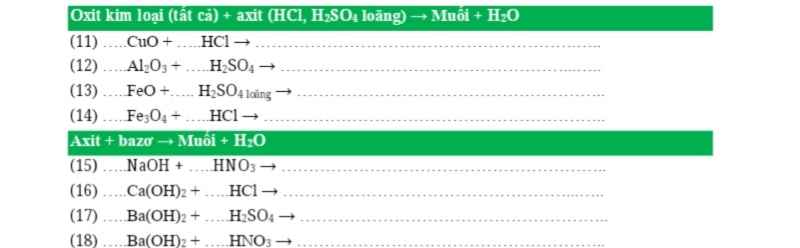
PTHH: \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
Tính theo sản phẩm
Ta có: \(\left\{{}\begin{matrix}n_{Al_2O_3}=\dfrac{30.6}{102}=0,3\left(mol\right)\\\Sigma n_{Al}=\dfrac{20}{27}\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Nhôm còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,45\left(mol\right)\\n_{Al\left(dư\right)}=\dfrac{19}{135}\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=0,45\cdot22,4=10,08\left(l\right)\\m_{Al\left(dư\right)}=\dfrac{19}{235}\cdot27\approx2,18\left(g\right)\end{matrix}\right.\)