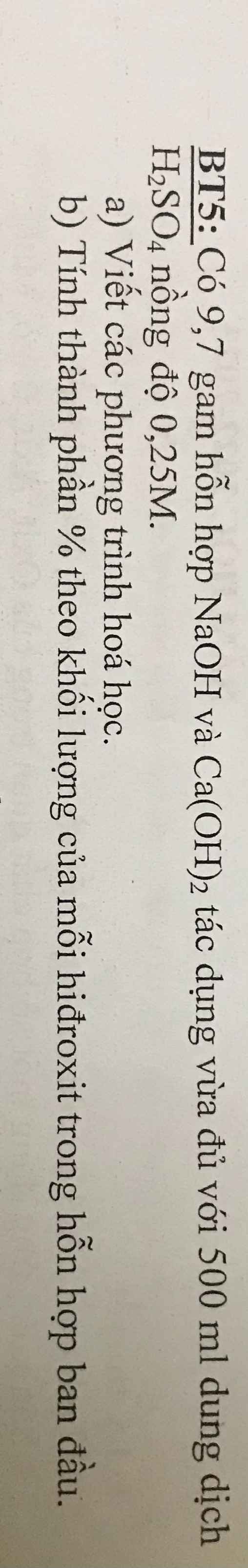\(n_{H_2SO_4}=0,5\times0,25=0,125\left(mol\right)\)
PT: \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\) (1)
x \(\dfrac{x}{2}\) (mol)
\(Ca\left(OH\right)_2+H_2SO_4\rightarrow CaSO_4+2H_2O\) (2)
y y (mol)
-Gọi x là \(n_{NaOH}\); y là \(n_{Ca\left(OH\right)_2}\).
-Ta có: \(\left\{{}\begin{matrix}\dfrac{x}{2}+y=0,125\\40x+74y=9,7\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,05\end{matrix}\right.\)
\(\%m_{NaOH}=\dfrac{0,15\times40\times100}{9,7}\approx61,86\%\)
\(\Rightarrow\%m_{Ca\left(OH\right)_2}=100\%-61,86\%=38,14\%\)