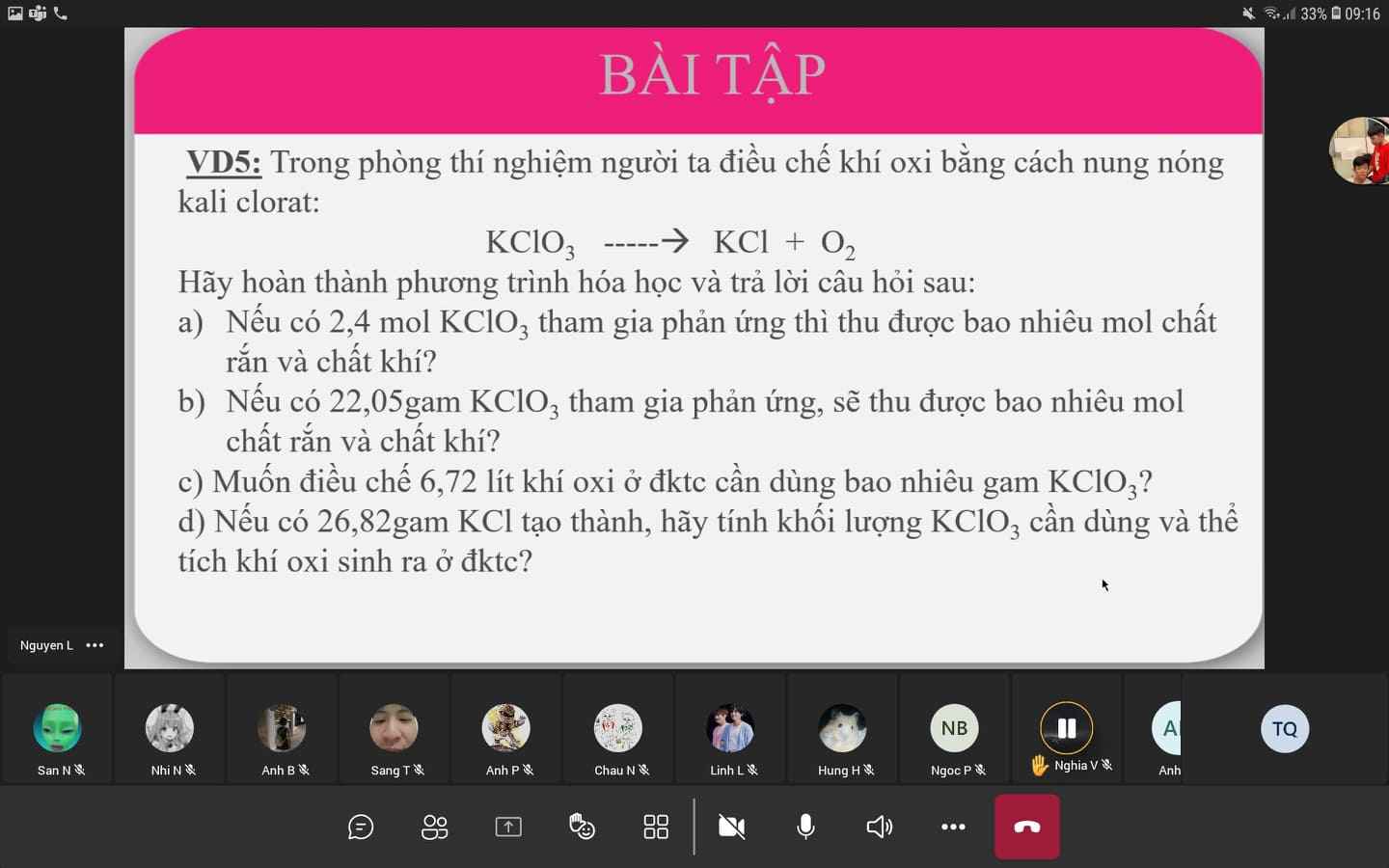
\(2KClO_3\underrightarrow{t^o,xt}2KCl+3O_2\)
a. \(n_{KCl}=n_{KClO_3}=2.4mol\)
\(n_{O_2}=\dfrac{3}{2}\times n_{KClO_3}=2.4\times\dfrac{3}{2}=3.6mol\)
b. \(n_{KClO_3}=\dfrac{22.05}{122.5}=0.18mol\)
\(n_{KCl}=n_{KClO_3}=0.18mol\)
\(n_{O_2}=\dfrac{3}{2}\times n_{KClO_3}=0.18\times\dfrac{3}{2}=0.27mol\)
c. \(n_{O_2}=\dfrac{6.72}{22.4}=0.3mol\)
\(n_{KClO_3}=\dfrac{2}{3}\times n_{O_2}=\dfrac{2}{3}\times0.3=0.2mol\Rightarrow m_{KClO_3}=0.2\times122.5=24.5g\)
d. \(n_{KCl}=\dfrac{26.82}{74.5}=0.36mol\)
\(n_{KCl}=n_{KClO_3}=0.36mol\Rightarrow m_{KClO_3}=0.36\times122.5=44.1g\)
\(n_{O_2}=\dfrac{2}{3}\times n_{KCl}=\dfrac{3}{2}\times0.36=0.54mol\Rightarrow V_{O_2}=0.54\times22.4=12.096l\)