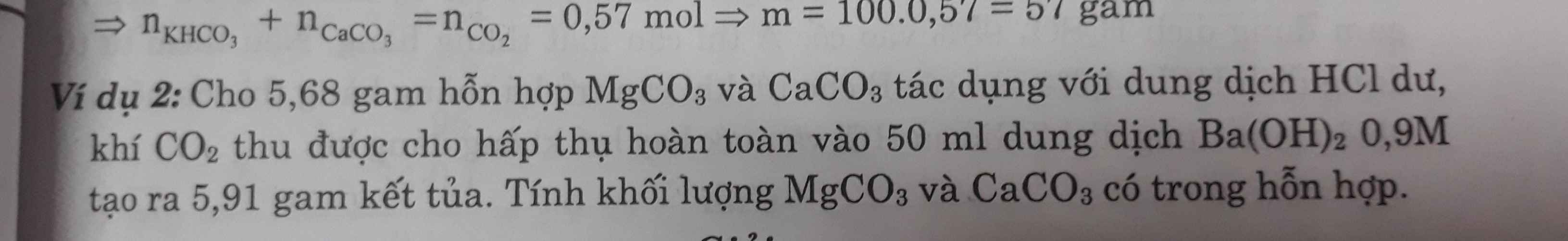PTHH:
CaCO3+2HCl--->CaCl2+H2O+CO2(1)
x__________________________x
MgCO3+2HCl---->MgCl2+H2O+CO2(2)
y_________________________y
CO2+Ba(OH)2--->BaCO3+H2O(3)
0,03__0,03________0,03
2CO2+Ba(OH)2---->Ba(HCO3)2 (4)
0,03____0,015
nBa(OH)2 bđ=0,045 mol
nBaCO3=0,03 mol
Theo pt (3): nCO2=nBaCO3=0,03 mol
=> nBa(OH)2 pu=0,03 mol
=>nBa(OH)2 dư=0,015 mol
=>nCO2(4)=0,03 mol
=>\(\Sigma\) nCO2=0,06 mol
Đặt nCaCO3=x mol;nMgCO3=y mol
Ta có hệ pt
\(\left\{{}\begin{matrix}100x+84y=5,68\\x+y=0,06\end{matrix}\right.\)
=> x=0,04 ; y=0,02
=> %CaCO3= 70,42% ; %MgCO3= 29,58%