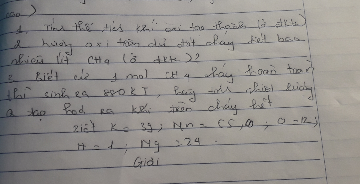a) \(n_{H_2O}=\frac{72}{18}=4mol\)
PTHH : \(2H_2+O_2\underrightarrow{đp}2H_2O\)
Theo PTHH : \(n_{O_2}=\frac{1}{2}n_{H_2O}=\frac{1}{2}4=2mol\)
=> \(V_{O_2}=2.2,24=4,48l\)
b) PTHH : \(4P+5O_2\underrightarrow{t^o}2P_2O_5\) (1)
Theo PTHH (1): \(n_{P_2O_5}=\frac{2}{5}n_{O_2}=\frac{2}{5}2=0,8mol\)
=> \(m_{P_2O_5}=0,8.142=113,6g\)
a, 2H2O--t0--> 2H2+ O2
Ta có nH2O=72/18=4
=> nO2=4/2=2
=> VO2=2.22,4=44,8l
b, 4P + 5O2--> 2P2O5
TA có nP2O5=2/5 .nO2=0,8
=> mP2O5=0,8.142=113,6g
nH2O = 72/18 = 4 mol
H2O -đp-> H2 + 1/2O2
4_______________2
VO2 = 2*22.4 = 44.8 l
4P + 5O2 -to-> 2P2O5
_____2_________0.8
mP2O5 = 0.8*142=113.6 g
2H2O --> 2H2 + O2
a) n\(H_2O\) = 72 / 18 =4 mol
Theo PTHH, ta có: n\(O_2\) = 4/2 = 2mol
V\(O_2\) = 2.22,4 = 44,8 l
b) 4P2 + 5O2 ---> 2P2O5
Theo PTHH, ta có: n\(P_2O_5\) = 0,8 mol
m\(P_2O_5\) = 0,8.142=113,6 g