\(a,PTHH:4K+O_2\underrightarrow{t^o}2K_2O\\ b,n_{O_2}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ Theo.PTHH:n_K=4n_{O_2}=4.0,1=0,4\left(mol\right)\\ \Rightarrow m_K=n.M=0,4.39=15,6\left(g\right)\\ c,Theo.PTHH:n_{K_2O}=2n_{O_2}=2.0,1=0,2\left(mol\right)\\ \Rightarrow m_{K_2O}=n.M=0,2.94=18,8\left(g\right)\)
Bài 26: Oxit
Đúng 1
Bình luận (0)
Các câu hỏi tương tự
Nung 94.8g KMnO4 a)Tính thể tích khí oxi thu được ở đktc nếu quá trình hao hụt 10%b)Dùng thể tích oxi thu được ở phần a để đốt cháy nhôm, tính khối lượng sản phẩm thu được
Phân hủy hoàn toàn 31,6g KMnO4 ( ở nhiệt độ cao )
1. Tính thể tích khí oxi tạo thành ( ở đktc )
2. Lượng oxi trên đủ đốt cháy hết bao nhiêu lít CH4 ( ở đktc )
3. Biết cứ 1 mol CH4 cháy hoàn toàn thì sinh ra 880KJ , hãy tính nhiệt lượng Q tạo ra hóa ra khí trên cháy hết
Biết K=39 , Mn=55, O=12, H=1, Mg=24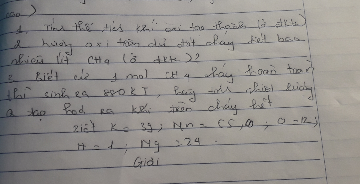
Cho hỗn hợp gồm Na và K tan hoàn trong nước thấy thoát ra 2,24 lít khí (đktc) a. Viết các PTHH xảy ra? b. Tính thành phần trăm khối lượng mỗi kim loại trong hỗn hợp? Biết trong hỗn hợp thì Kali có khối lượng là 3,9 gam
Đốt cháy hoàn toàn 5,4 g kim loại nhôm Al trong khí oxi. a. Tính thể tích khí oxi (đktc) cần dùng cho phản ứng. b. Tính khối lượng KClO3 cần dùng để điều chế lượng oxi trên.
đốt cháy hoàn toàn 10.8 gam nhôm trong bình đựng khí oxi sau phản ứng thu được nhôm oxit
a.viết phản ứng hóa học của phản ứng trên(viết pt tính số mol nha )
b.tính khối lượng nhôm oxit tạo thành sau pahnr ứng
c.tính thể tích oxi cần dùng (dktc)
d. để có lượng oxi trên cần nhiệt phân ít nhất bao nhiêu gam KClO3
Đốt cháy 6,4 g lưu huỳnh trong một bình chứa 11,2 lít không khí ( chứa 20% thể tích khí oxi) ( đktc). Tính khối lượng khí sunfurơ thu được.
Đốt cháy hỗn hợp bột của kim loại magie và nhôm cần 33,6 lít khí oxi ở đktc. Biết khối lượng nhôm trong hỗn hợp là 2,7 gam. Hãy tính thành phần phần trăm khối lượng của hai kim loại trong hỗn hợp trên.
Đốt cháy hoàn toàn 10,85g photpho trong không khí.
a. Tính khối lượng oxit tạo thành.
b. Tính thể tích không khí đã dùng.
c*. Tính khối lượng KMnO4 cần dùng để điều chế được thể tích khí oxi ở trên.
Người ta đốt sắt trong khí oxi, sau phản ứng thu được 13,92 gam oxit sắt từ (Fe3O4Fe3O4).
a. Viết phản ứng hóa học của phản ứng trên.
B. Tính khối lượng sắt đã tham gia phản ứng.
c. Tính thể tích oxi cần dùng (đktc).
d. Để có lượng oxi trên cần nhiệt phân ít nhất bao nhiêu gam KMnO4KMnO4.











