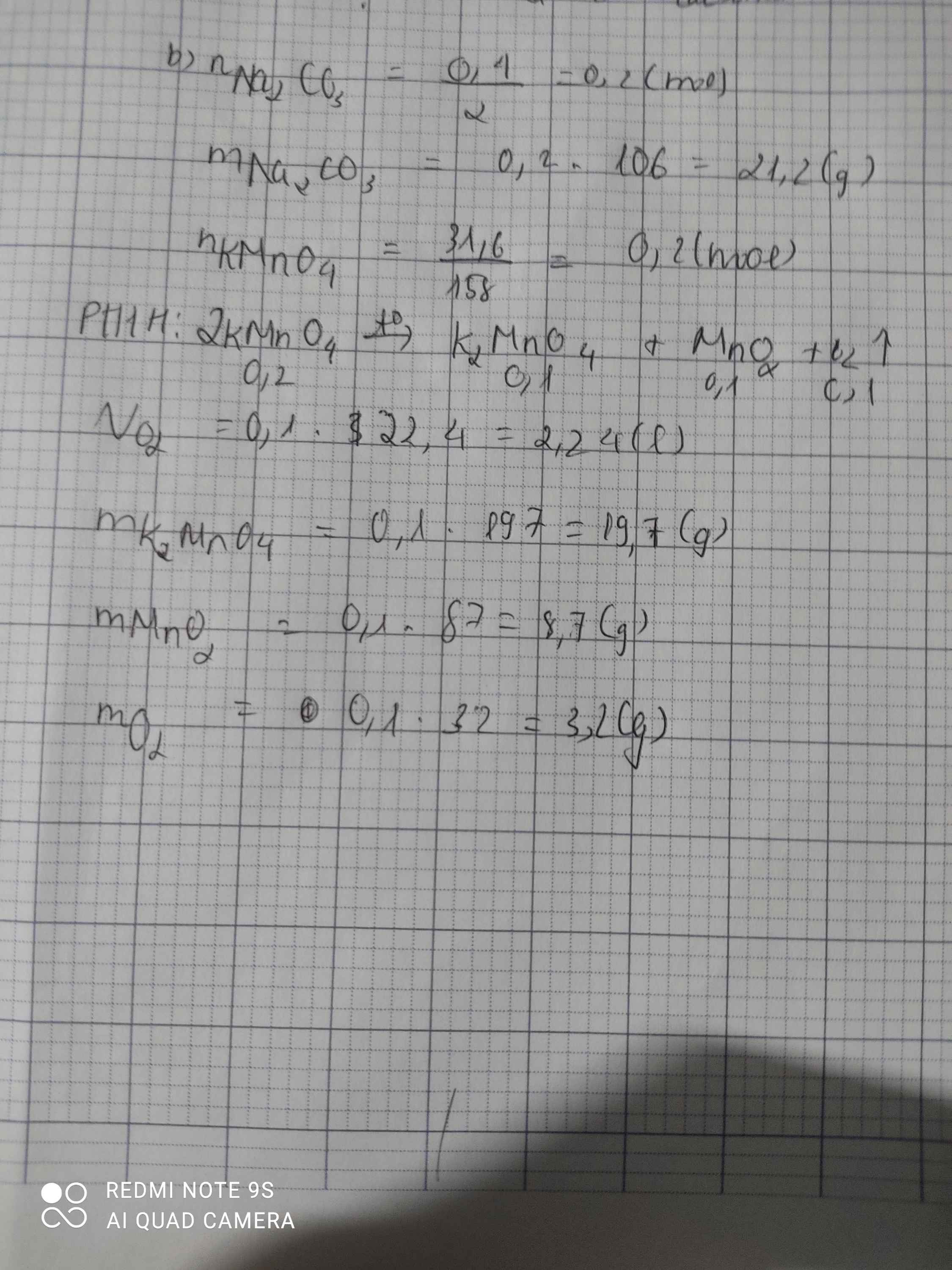\(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
\(2KMnO_4->K_2MnO_4+MnO_2+O_2\)
2......................1................1...................1
0,2................0,1..............0,1....................0,1
\(m_{K_2MnO_4}=0,1.197=19,7\left(g\right)\\ m_{MnO_2}=0,1.87=8,7\left(g\right)\\ m_{O_2}=0,1.32=3,2\left(g\right)\)
2KMnO4-to>K2MnO4+MnO2+O2
0,2---------------0,1----------0,1-----0,1 mol
n KMnO4=\(\dfrac{31,6}{158}\)=0,2 mol
=>VO2=0,1.22,4=2,24l
=>m MnO2=0,1.87=8,7g
=>m K2MnO4=0,1.197=19,7g