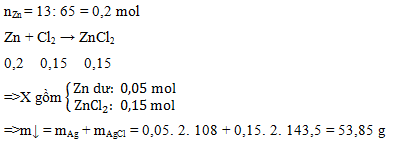\(Zn + Cl_2 \xrightarrow{t^o} ZnCl_2\\ n_{Zn} = \dfrac{13}{65} = 0,2 > n_{Cl_2} = 0,15\). Do đó.Zn dư
Zn + Cl2 \(\xrightarrow{t^o}\) ZnCl2
0,15.......0,15....0,15......................(mol)
Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
0,05........................................... 0,1..........(mol)
ZnCl2 + 2AgNO3 → 2AgCl + Zn(NO3)2
0,15...............................0,3.........................(mol)
Vậy :
\(m_{kết\ tủa} = 0,1.108 + 0,3.143,5 = 53,85(gam)\)
\(n_{Zn}=\dfrac{13}{65}=0.2\left(mol\right)\)
\(Zn+Cl_2\underrightarrow{t^0}ZnCl_2\)
\(0.15....0.15...0.15\)
\(\Rightarrow Zndư,Cl_2hết\)
\(ZnCl_2+2AgNO3\rightarrow Zn\left(NO_3\right)_2+2AgCl\)
\(0.15..................................................0.3\)
\(m_{AgCl}=0.3\cdot143.5=430.05\left(g\right)\)
Chúc bạn học tốt !!!