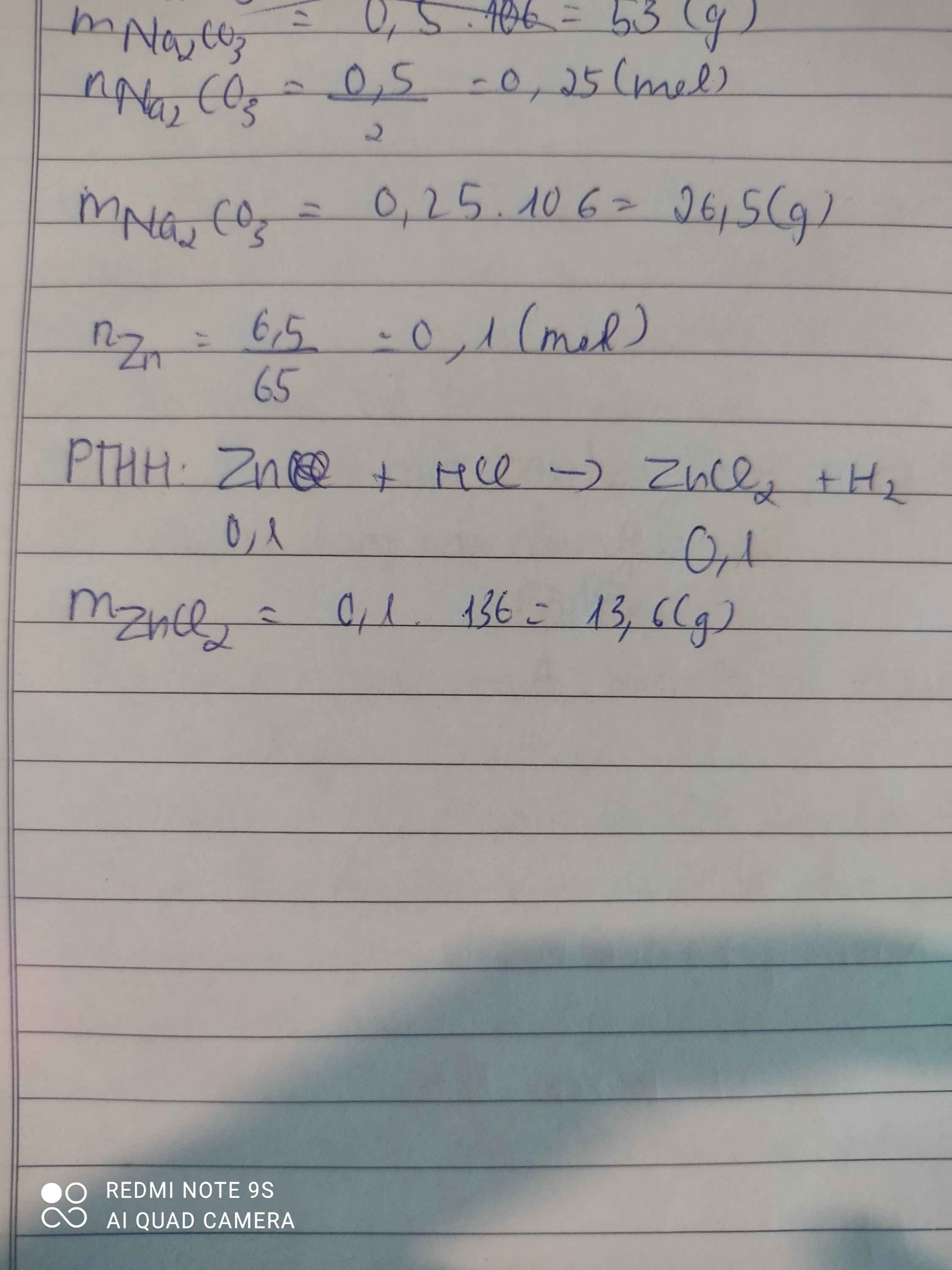\(Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ n_{ZnCl_2}=n_{Zn}=0,1\left(mol\right)\\ \Rightarrow m_{ZnCl_2}=0,1.136=13,6\left(g\right)\)
\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,1\left(mol\right)\\ \Rightarrow m_{H_2}=2.0,1=0,2\left(g\right);m_{ZnCl_2}=136.0,1=13,6\left(g\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
1 2 1 1 ( mol )
0,1 0,1 ( mol )
\(n_{Zn}=\dfrac{m}{M}=\dfrac{6,5}{65}=0,1mol\)
\(m_{ZnCl_2}=n.M=0,1.136=13,6g\)