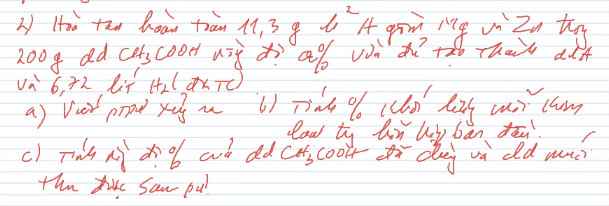a, PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
\(Zn+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2\)
b, Gọi: \(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Zn}=y\left(mol\right)\end{matrix}\right.\) ⇒ 24x + 65y = 11,3 (1)
Có: \(n_{H_2}=0,3\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Mg}+n_{Zn}=x+y\left(mol\right)\)
⇒ x + y = 0,3 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,2\left(mol\right)\\y=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,2.24}{11,3}.100\%\approx42,48\%\\\%m_{Zn}\approx57,52\%\end{matrix}\right.\)
c, Có: \(n_{CH_3COOH}=2n_{H_2}=0,6\left(mol\right)\)
\(\Rightarrow m_{CH_3COOH}=0,6.60=36\left(g\right)\)
\(\Rightarrow C\%_{CH_3COOH}=\dfrac{36}{200}.100\%=18\%\)
⇒ m dd sau pư = 11,3 + 200 - 0,3.2 = 210,7 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{\left(CH_3COO\right)_2Mg}=\dfrac{0,2.142}{210,7}.100\%\approx13,48\%\\C\%_{\left(CH_3COO\right)_2Zn}=\dfrac{0,1.183}{210,7}.100\%\approx8,68\%\end{matrix}\right.\)
Bạn tham khảo nhé!
a) \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Mg + 2CH3COOH ------> (CH3COO)2Mg + H2
Mol: x 2x x x
Zn + 2CH3COOH ------> (CH3COO)2Zn + H2
Mol: y 2y y y
Ta có: \(\left\{{}\begin{matrix}24x+65y=11,3\\x+y=0,3\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\left(mol\right)\\y=0,1\left(mol\right)\end{matrix}\right.\)
b, \(\%m_{Mg}=\dfrac{0,2.24.100\%}{11,3}=42,48\%\)
\(\%m_{Zn}=100\%-42,48\%=57,5\%\%\)
c, \(n_{CH_3COOH}=2.0,2+2.0,1=0,6\left(mol\right)\)
\(C\%_{ddCH_3COOH}=\dfrac{0,6.60.100\%}{200}=18\%\)
\(m_{dd.sau.pứ}=11,3+200-0,3.2=210,7\left(g\right)\)
\(C\%_{dd\left(CH_3COOH\right)_2Mg}=\dfrac{0,2.144.100\%}{210,7}=13,67\%\)
\(C\%_{dd\left(CH_3COOH\right)_2Zn}=\dfrac{0,1.185.100\%}{210,7}=8,78\%\)