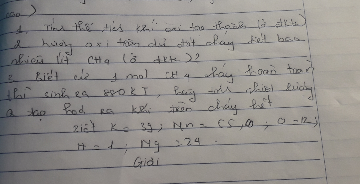\(n_C=\dfrac{m}{M}=\dfrac{3,6}{12}=0,3\left(mol\right)\\ PTHH\left(1\right):C+O_2\underrightarrow{t^o}CO_2\\ Theo.PTHH\left(1\right):n_O=n_C=0,3\left(mol\right)\)
\(PTHH\left(2\right):2KClO_3\underrightarrow{t^o}2KCl+3O_2\\ Theo.PTHH\left(2\right):n_{KClO_3}=\dfrac{2}{3}n_O=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ \Rightarrow m_{KClO_3}=n.M=0,2.122,5=24,5\left(g\right)\)