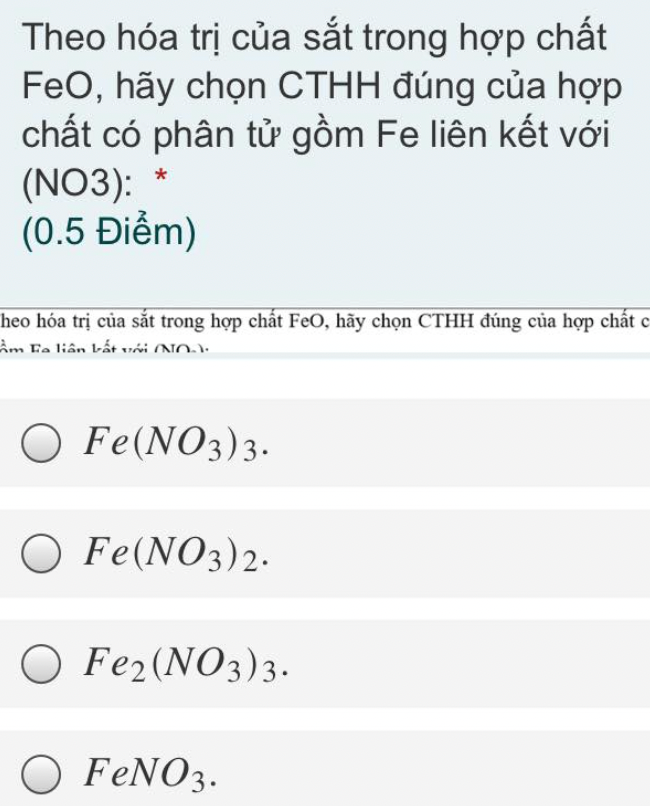
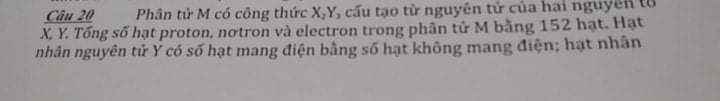\(d_{\dfrac{A}{H_2}}=32\\ M_A=32.2=64\left(\dfrac{g}{mol}\right)\)
\(m_S=\dfrac{50.64}{100}=32g\\ m_O=64-32=32g\\ n_S=\dfrac{32}{32}=1mol\\ n_O=\dfrac{32}{16}=2mol\\ CTHH:SO_2\\ \Rightarrow B\)
\(d_{\dfrac{A}{H_2}}=32\\ M_{H_2}=2\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_A=d_{\dfrac{A}{H_2}}.M_{H_2}=32.2=64\left(\dfrac{g}{mol}\right)\\ m_S=\%S.M_A=50\%.64=32\left(g\right)\\ m_O=m_A-m_S=64-32=32\left(g\right)\\ n_S=\dfrac{m}{M}=\dfrac{32}{32}\left(mol\right)\\ n_O=\dfrac{32}{16}=2\left(mol\right)\\ CTHH:SO_2\Rightarrow B\)