\({{\rm{n}}_{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COOH}}}}{\rm{ = }}\frac{{{\rm{16,2}}}}{{{\rm{60}}}}{\rm{ = 0,27 (mol); }}{{\rm{n}}_{{{\rm{C}}_5}{{\rm{H}}_{12}}{\rm{O}}}}{\rm{ = }}\frac{{{\rm{15,2}}}}{{88}}{\rm{ }} \approx {\rm{ 0,173 (mol)}}\)
Phương trình hóa học:
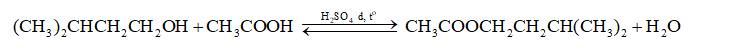
Mol: 0,173 0,27
Ta có: \(\frac{{0,173}}{1} < \frac{{0,27}}{1}\) => isoamyl alcohol hết, ester tính theo isoamyl alcohol.
\(\begin{array}{l}{{\rm{n}}_{{{\rm{C}}_6}{{\rm{H}}_{12}}{{\rm{O}}_2}{\rm{ (ester)}}}}{\rm{ = }}{{\rm{n}}_{{{\rm{C}}_5}{{\rm{H}}_{12}}{\rm{O}}}}{\rm{ = 0,173 (mol) }}\\ \Rightarrow {{\rm{m}}_{{{\rm{C}}_6}{{\rm{H}}_{12}}{{\rm{O}}_2}{\rm{ (ester)}}}} = {\rm{0,173}} \times 116{\rm{ = 20,068 (g)}}\\ \Rightarrow {\rm{H = }}\frac{{14,16}}{{20,068}} \times 100\% \approx 71\% \end{array}\)


