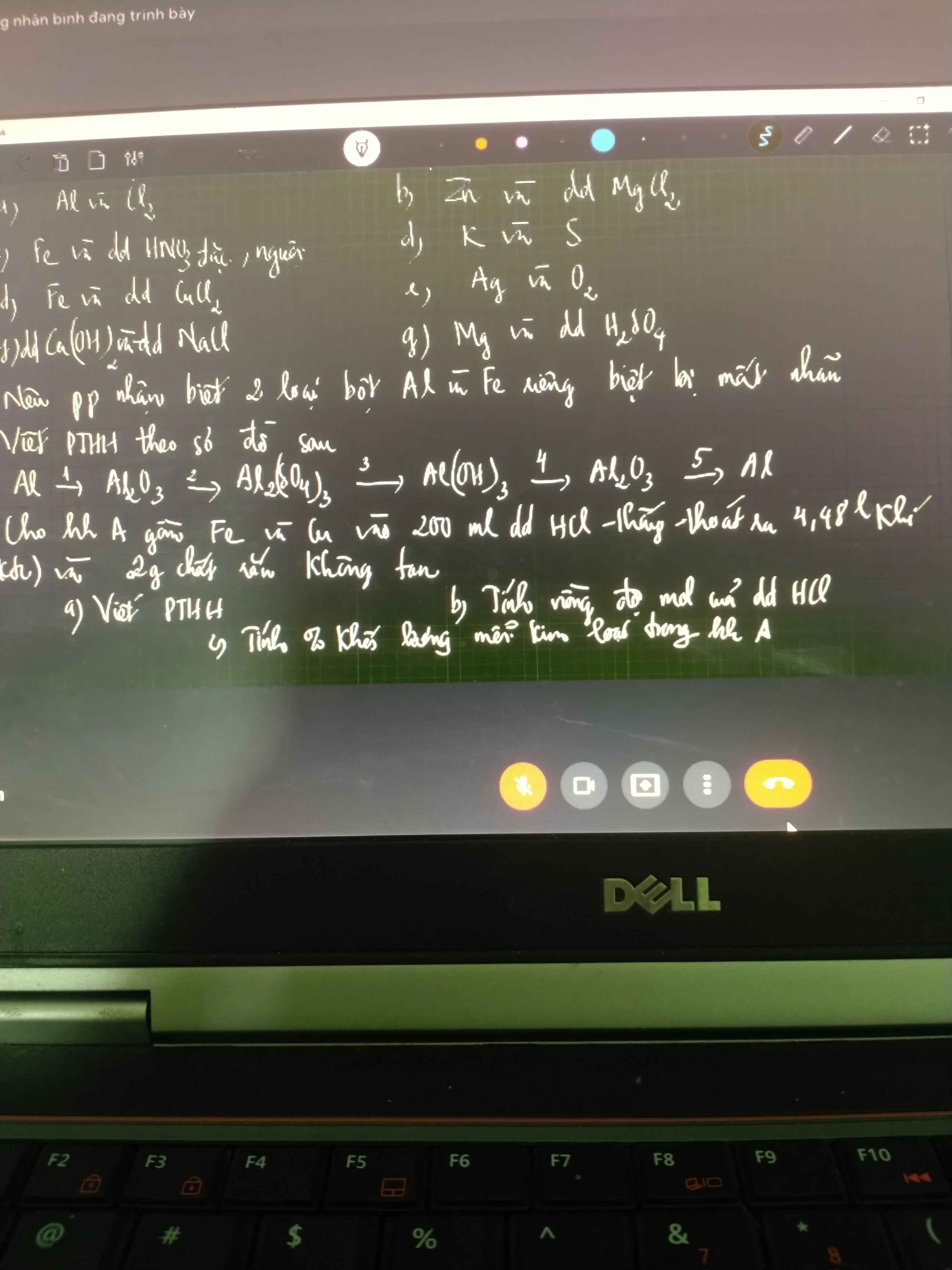
Câu 4:
Chất rắn ko tan là Cu
\(\Rightarrow m_{Cu}=2(g)\\ n_{H_2}=\dfrac{4,48}{22,4}=0,2(mol)\\ a,PTHH:Fe+2HCl\to FeCl_2+H_2\\ b,n_{HCl}=2n_{H_2}=0,4(mol)\\ \Rightarrow C_{M_{HCl}}=\dfrac{0,4}{0,2}=2M\\ c,n_{Fe}=n_{H_2}=0,2(mol)\\ \Rightarrow m_{Fe}=0,2.56=11,2(g)\\ \Rightarrow \%_{Fe}=\dfrac{11,2}{11,2+2}.100\%\approx 84,85\%\\ \Rightarrow \%_{Cu}=100\%-84,85\%=15,15\%\)
Vì Cu ko pư vs Hcl nên chất rắn ko tan ở đây là cu
nH2=0,2mol
Fe+2Hcl--->FeCl2+H2
=>mFe=0,2*56=11,2
CMHcl=0,4/0,2=2M
%Fe=11,2/13,2*100%=84,84%
%Cu=100-84,84=15,16%
Câu 3:
\((1)4Al+3O_2\xrightarrow{t^o}2Al_2O_3\\ (2)Al_2O_3+3H_2SO_4\to Al_2(SO_4)_3+3H_2O\\ (3)Al_2(SO_4)_3+6NaOH\to 2Al(OH)_3\downarrow+3Na_2SO_4\\ (4)2Al(OH)_3\xrightarrow{t^o}Al_2O_3+3H_2O\\ (5)2Al_2O_3\xrightarrow{đpnc}4Al+3O_2\)