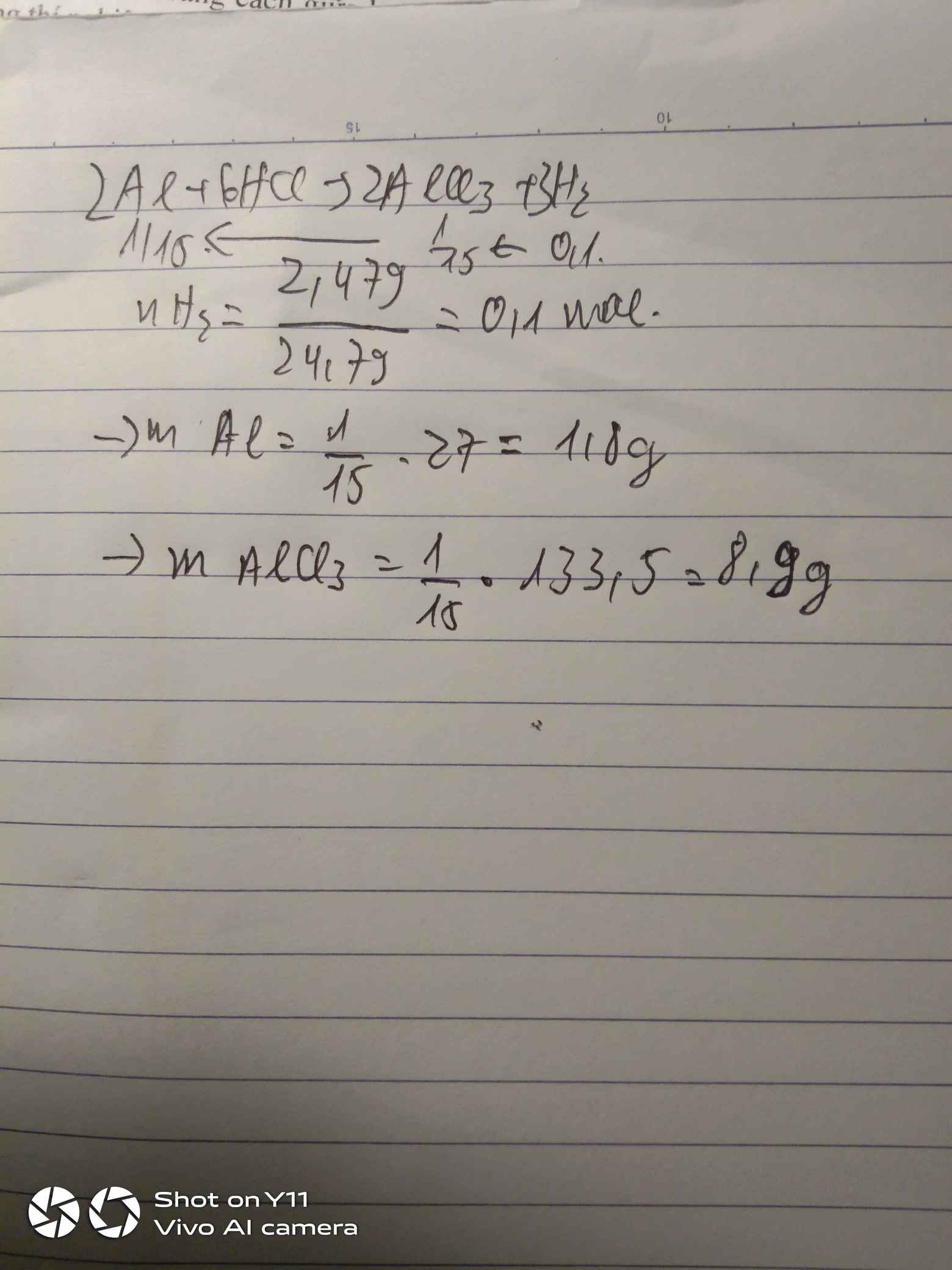\(n_{H_2\left(đkc\right)}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\\ 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ n_{Al}=n_{AlCl_3}=\dfrac{2}{3}.n_{H_2}=\dfrac{2}{3}.0,1=\dfrac{1}{15}\left(mol\right)\\ \Rightarrow m_{Al}=27.\dfrac{1}{15}=1,8\left(g\right)\\ m_{AlCl_3}=\dfrac{1}{15}.133,5=8,9\left(g\right)\)
\(n_{H_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
\(\dfrac{1}{15}\)<--------------\(\dfrac{1}{15}\)<-----0,1
=> \(m_{Al}=\dfrac{1}{15}.27=1,8\left(g\right)\)
=> \(m_{AlCl_3}=\dfrac{1}{15}.133,5=8,9\left(g\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\\ n_{Al}=n_{AlCl_3}=\dfrac{2}{3}n_{H_2}=\dfrac{2}{3}.\dfrac{2,497}{24,79}=\dfrac{1}{15}\left(mol\right)\\ \Rightarrow m_{Al}=\dfrac{1}{15}.27=1,8\left(g\right);m_{AlCl_3}=\dfrac{1}{15}.133,5=8,9\left(g\right)\)