\(Metan:CH_4\\ But-1-en:CH_2=CH-CH_2-CH_3\\ n_{Br_2}=\dfrac{16}{160}=0,1\left(mol\right)\\ C_4H_8+Br_2\rightarrow C_4H_8Br_2\\ n_{C_4H_8}=n_{Br_2}=0,1\left(mol\right)\\ \%m_{C_4H_8}=\dfrac{56.0,1}{7,2}.100\approx77,778\%\\ \Rightarrow\%m_{CH_4}\approx22,222\%\\ n_{CH_4}=\dfrac{7,2-0,1.56}{16}=0,1\left(mol\right)\\ CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\\ C_4H_8+6O_2\rightarrow\left(t^o\right)4CO_2+4H_2O\)
Chương 1. Sự điện li
Đúng 2
Bình luận (0)
Các câu hỏi tương tự
Đốt cháy hoàn toàn hỗn hợp X gồm 3,2 gam metan và 2,8 gam etilen rồi dẫn sản
phẩm cháy vào bình đựng nước vôi trong dư thấy khối lượng dung dịch trong
binh giảm m gam so với ban đấu. Tính giá trị của m?
Đốt cháy hoàn toàn 2,24 lít (đktc) một ankan A, sau phản ứng thu được 20,4 gam hỗn hợp sản phẩm khí và hơi.a. Xác định công thức phân tử của A.b. Hỗn hợp khí Z gồm ankan A và một hiđrocacbon B. Lấy 2,24 lít hỗn hợp Z rồi dẫn từ từ cho đến hết vào một lượng dư dung dịch AgNO3 trong NH3 thấy xuất hiện m gam kết tủa. Mặt khác, nếu đốt cháy hoàn toàn 2,24 lít hỗn hợp Z thì thu được 5,824 lít khí CO2. Các thể tích khí đều đo ở đktc. Tính m.
Đọc tiếp
Đốt cháy hoàn toàn 2,24 lít (đktc) một ankan A, sau phản ứng thu được 20,4 gam hỗn hợp sản phẩm khí và hơi.
a. Xác định công thức phân tử của A.
b. Hỗn hợp khí Z gồm ankan A và một hiđrocacbon B. Lấy 2,24 lít hỗn hợp Z rồi dẫn từ từ cho đến hết vào một lượng dư dung dịch AgNO3 trong NH3 thấy xuất hiện m gam kết tủa. Mặt khác, nếu đốt cháy hoàn toàn 2,24 lít hỗn hợp Z thì thu được 5,824 lít khí CO2. Các thể tích khí đều đo ở đktc. Tính m.
Hỗn hợp X gồm axetilen và 2 ank – 1 – in. Cho m gam hỗn hợp X tác dụng với lượng dư dung dịch AgNO3/ NH3 thu được m + 55,64 gam hỗn hợp kết tủa. Mặt khác đốt m gam hỗn hợp X thu được 55,44 gam CO2 và 15,48 gam nước. Tính phần trăm khối lượng axetilen trong hỗn hợp.
Phương trình số 2 làm sao có được v mm?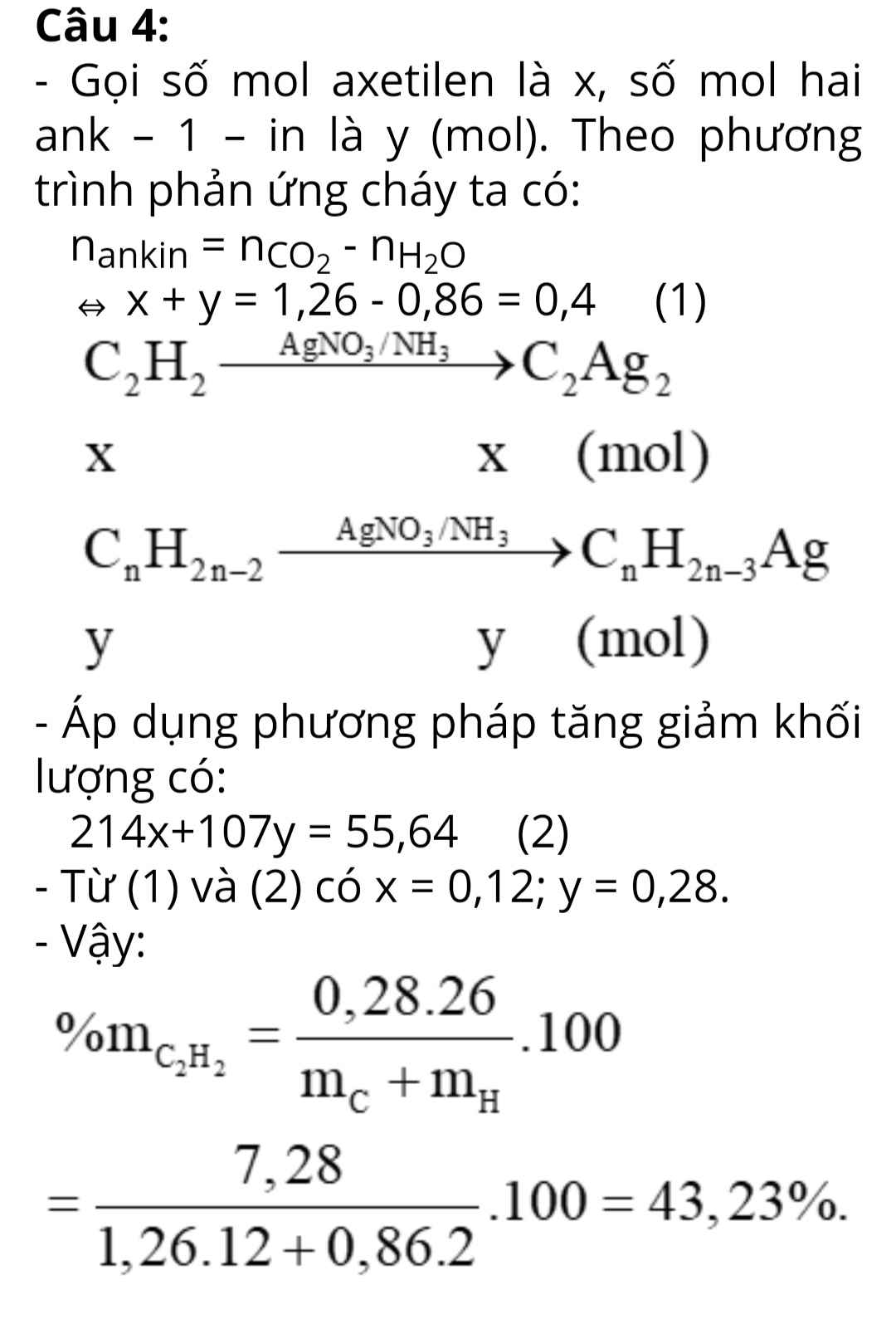
Câu 8:Cho m gam hỗn hợp gồm (Zn, Cu) tác dụng hết với dung dịch HNO3 dư thu được ddA và hỗn hợp khí (N2, NO, NO2, N2O). Thấy khối lượng nước có trong dung dịch tăng lên 18 gam. Cho dung dịch A tác dụng với dung dịch NaOH dư, không thấy khí thoát ra. Số mol HNO3 đã tham gia phản ứng là:
Cho m gam hỗn hợp X gồm Na và Al4C3 ( tỉ lệ mol tương ứng là 4:1) vào nước dư thu được dung dịch X và hỗn hợp khí Y. Sục khí CO2 dư vào dung dịch X thu được 31,2 gam kết tủa. Tính giá trị của m?
Cho m gam hỗn hợp X gồm (Zn, Fe) tác dụng hết với dung dịch HNO3 dư thu được ddA và hỗn hợp khí (NO, NO2). Cho dung dịch A tác dụng với dung dịch NaOH dư, thu được chất rắn B, nung chất rắn B trong chân không đến khối lượng không đổi được 16 gam chất rắn C. Khối lượng của Fe trong hỗn hợp X là
Hoà tan m gam hỗn hợp Na2SO4 và CuSO4 vào nước được dung dịch A. Cho dung dịch A phản ứng hoàn toàn với dung dịch ba(no3)2 thì thu được 13,98 gam kết tủa.
Mặt khác khi cho cùng lượng hỗn hợp trên tác dụng với dung dịch KOH thì được 1,96 g kết tủa Tính phần trăm khối lượng của mỗi muối trong hỗn hợp ban đầu
Hòa tan hoàn toàn hỗn hợp gồm 3,4 gam NaNO3 và 5,22 gam Ba(NO3)2 vào nước để được
500ml dung dịch A. Tính nồng độ mol/l của các ion có trong dung dịch A
Oxi hoá chậm m gam Fe ngoài không khí thu được 12g hỗn hợp A gồm FeO, Fe2O3, Fe3O4 và Fe dư. Hoà tan hỗn hợp A vừa đủ bởi dung dịch HNO3 thu được 2,24 lít NO duy nhất (đktc). Khối lượng m gam Fe ban đầu là:








