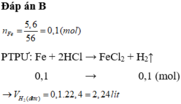\(n_{Fe}=\dfrac{1,12}{56}=0,02\left(mol\right)\\
n_{Cu}=\dfrac{1,6}{64}=0,025\left(mol\right)\\
pthh:Fe+2HCl\rightarrow FeCl_2+H_2\)
0,02 0,02
\(Cu+HCl\) không phản ứng
\(V_{H_2}=0,02.22,4=0,448l\)
Fe+2HCl->Fecl2+H2
0,02------------------0,02
=>VH2=0,02.22,4=0,448l