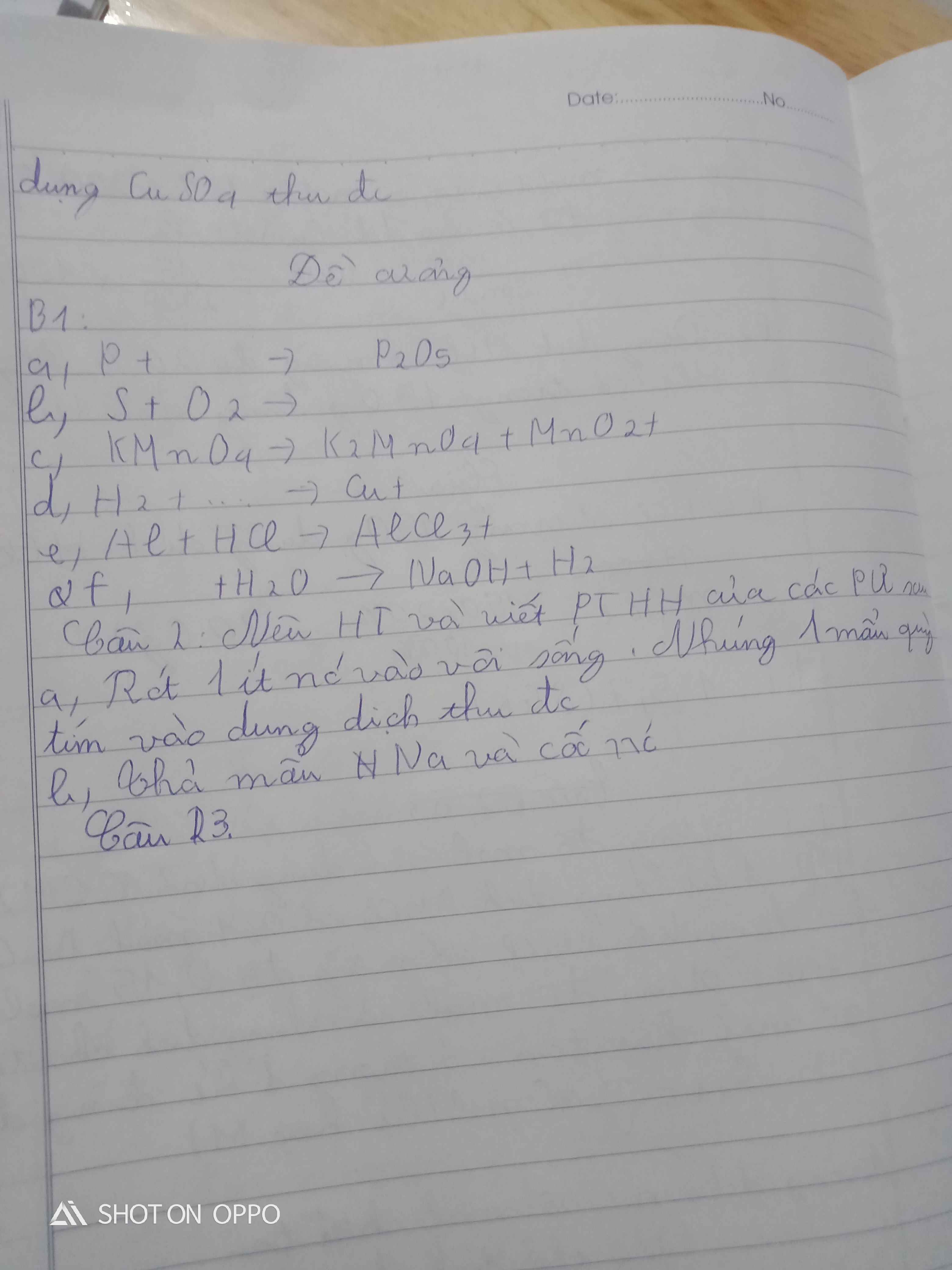Al2O3+6HCl->2AlCl3+3H2O
0,1------0,6-------0,2---------0,3
n Al2O3=0,1 mol
=>m HCl=0,6.36,5=21,9g
=>mAlCl3=0,2.133,5=26,7g
a, PT: \(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
b, Có: \(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
Theo PT: \(n_{HCl}=6n_{Al_2O_3}=0,6\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\)
c, Theo PT: \(n_{AlCl_3}=2n_{Al_2O_3}=0,2\left(mol\right)\Rightarrow m_{AlCl_3}=0,2.133,5=26,7\left(g\right)\)
\(n_{H_2}=3n_{Al_2O_3}=0,3\left(mol\right)\Rightarrow n_{H_2}=0,3.2=0,6\left(mol\right)\)
Bạn tham khảo nhé!