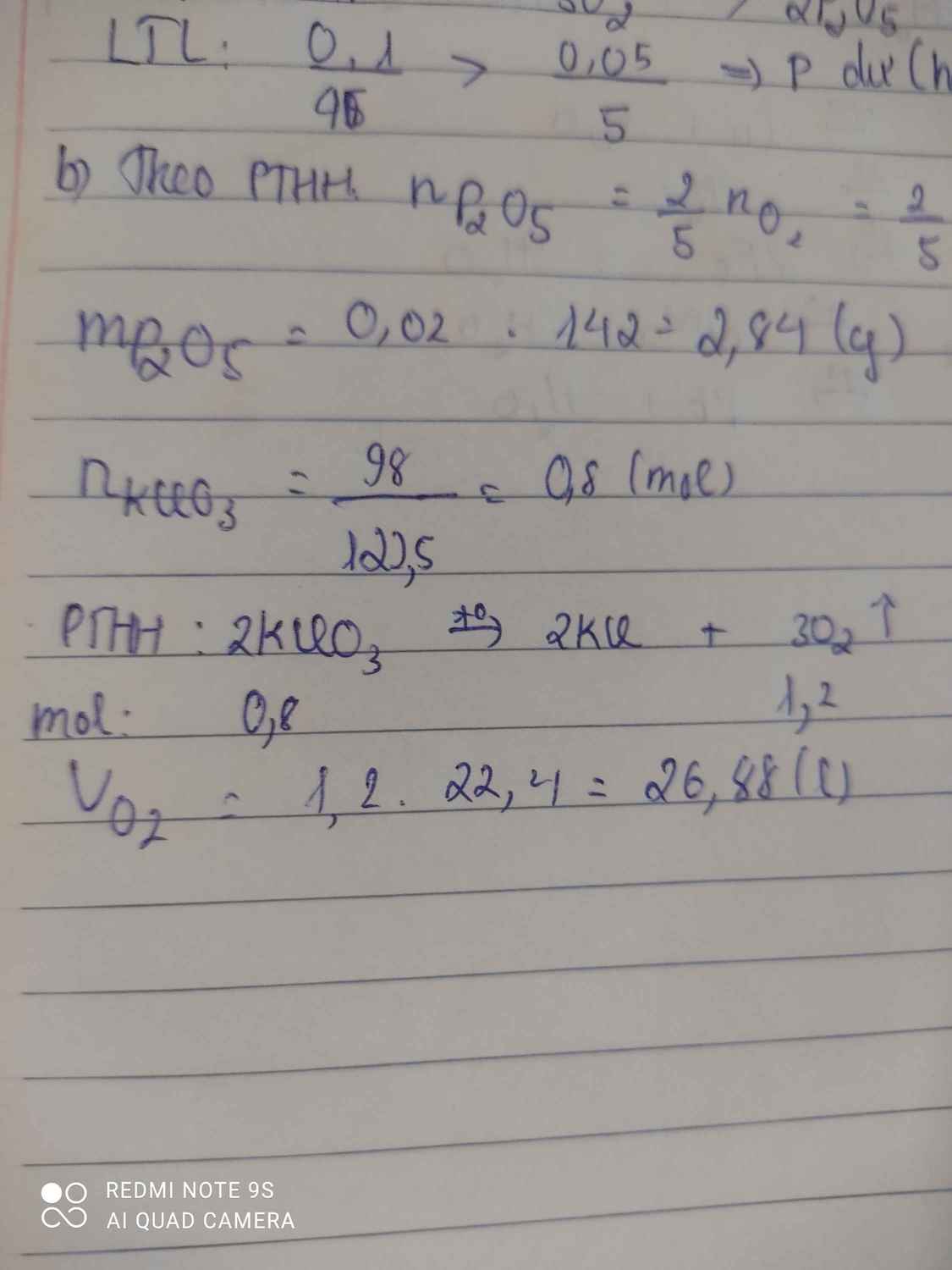\(n_{KClO_3}=\dfrac{98}{122,5}=0,8\left(mol\right)\)
PTHH : 2KClO3 -> 2KCl + 3O2
0,8 1,2
\(V_{O_2}=1,2.22,4=26,88\left(l\right)\)
\(2KClO_3\rightarrow2KCl+3O_2\)
\(n_{KClO_3}=\dfrac{m}{M}=\dfrac{98}{122,5}=0.8mol\)
\(V_{O_2}=n_{o_2}.22,4=\left(\dfrac{0,8.3}{2}\right).22,4=26,88l\)