Câu 4:
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)\\ Na+H_2O\to NaOH+\dfrac{1}{2}H_2\\ Na_2O+H_2O\to 2NaOH\\ \Rightarrow n_{Na}=2n_{H_2}=0,2(mol)\\ a,\%_{Na}=\dfrac{0,2.23}{10,8}.100\%=42,59\%\\ \%_{Na_2O}=100\%-42,59\%=57,41\%\\ b,n_{Na_2O}=\dfrac{10,8-0,2.23}{62}=0,1(mol)\\ \Rightarrow \Sigma n_{NaOH}=0,2+0,2=0,4(mol)\\ \Rightarrow m_{NaOH}=0,4.40=16(g)\)
Câu 5:
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)\\ R+H_2O\to ROH+\dfrac{1}{2}H_2\\ R_2O+H_2O\to 2ROH\\ \Rightarrow n_{R}=2n_{H_2}=0,2(mol)\\ \Rightarrow n_{R_2O}=0,1(mol)\\ \Rightarrow M_R.0,2+(2M_R+16).0,1=10,8\\ \Rightarrow M_R=23(g/mol)\)
Vậy R là Na

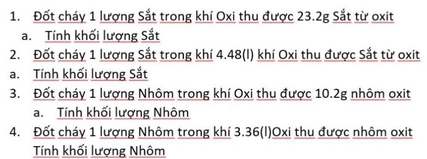
 mng ơi giúp mih với, mih cảm ơn nhiều ạ
mng ơi giúp mih với, mih cảm ơn nhiều ạ