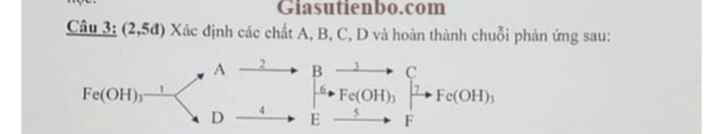
Câu 3.1
Gọi số mol CO2 sinh ra là a (mol)
PTHH: Ca(OH)2 + CO2 --> CaCO3 + H2O
a-------->a
mgiảm = mCaCO3 - mCO2
=> 100a - 44a = 6,72
=> a = 0,12 (mol)
PTHH: FexOy + yCO --to--> xFe + yCO2
\(\dfrac{0,12}{y}\)<----------------------0,12
=> \(M_{Fe_xO_y}=56x+16y=\dfrac{6,96}{\dfrac{0,12}{y}}=58y\left(g/mol\right)\)
=> \(\dfrac{x}{y}=\dfrac{3}{4}\) => CTHH: Fe3O4
\(n_{Fe_3O_4}=\dfrac{6,96}{232}=0,03\left(mol\right)\)
=> nFe = 0,09 (mol)
\(\left\{{}\begin{matrix}n_{AgNO_3}=1,2.0,1=0,12\left(mol\right)\\n_{Cu\left(NO_3\right)_2}=0,6.0,1=0,06\left(mol\right)\end{matrix}\right.\)
PTHH: Fe + 2AgNO3 --> Fe(NO3)2 + 2Ag
0,06<--0,12-------------------->0,12
Fe + Cu(NO3)2 --> Fe(NO3)2 + Cu
0,03-->0,03----------------------->0,03
=> Rắn C gồm \(\left\{{}\begin{matrix}Ag:0,12\left(mol\right)\\Cu:0,03\left(mol\right)\end{matrix}\right.\)
=> m = 0,12.108 + 0,03.64 = 14,88 (g)







 giúp em 2 câu này vs ạ ngày mai nộp rồi nhưng vẫn ko bt cách làm.Em cảm ơn trc ạ
giúp em 2 câu này vs ạ ngày mai nộp rồi nhưng vẫn ko bt cách làm.Em cảm ơn trc ạ