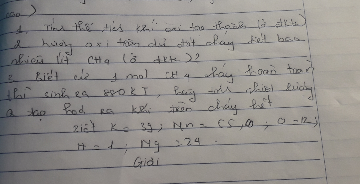\(n_{CH4}=\frac{4,48}{33,4}=0,2\left(mol\right)\)
PTHH: CH4 + 2O2 --> CO2 + 2H2O
0,2 -> 0,4 (mol)
=> \(V_{O2}=0,4.22,4=8,96\left(l\right)\)
=> \(V_{kk}=8,96:20\%=44,8\left(l\right)\)
\(CH_4+2O_2\rightarrow CO_2+2H_2O\)
0,2____0,4_______________
\(n_{CH4}=\frac{4,48}{22,4}=0,2\left(mol\right)\)
\(V_{O2}=0,4.22,4=8,96\left(l\right)\)
Ta có :
\(V_{O2}=20\%_{kk}\)
\(\Leftrightarrow V_{kk}=\frac{8,96}{20\%}=44,8\left(l\right)\)