\(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
PTHH: 2Al + 6HCl ---> 2AlCl3 + 3H2
0,1 0,1 0,15
\(\rightarrow\left\{{}\begin{matrix}m_{AlCl_3}=0,1.133,5=13,35\left(g\right)\\V_{H_2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\end{matrix}\right.\)
Đúng 1
Bình luận (0)










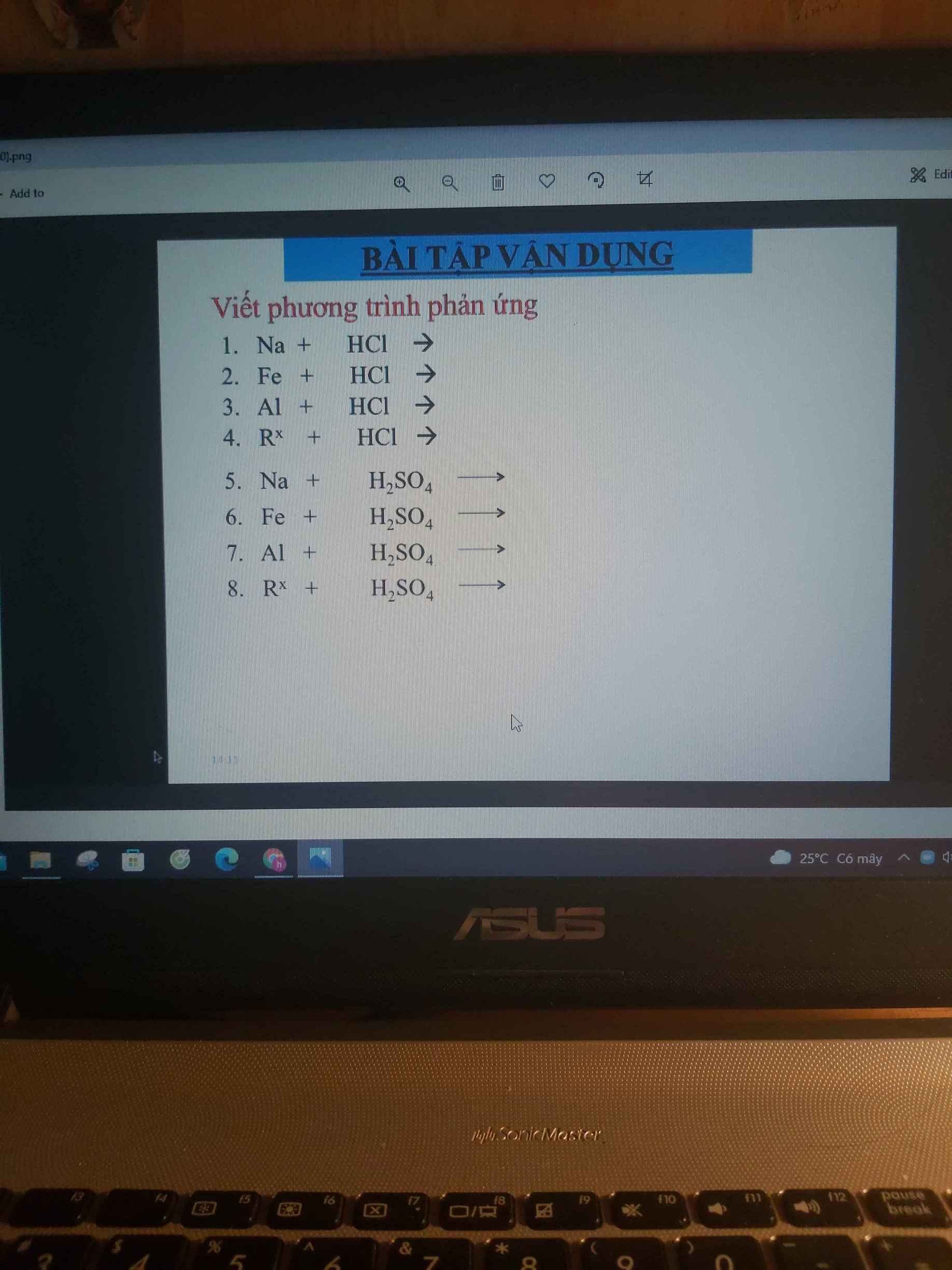

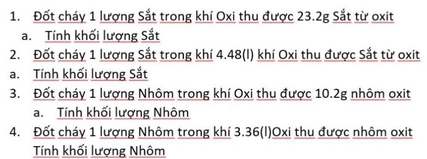


 mng ơi giúp mih với, mih cảm ơn nhiều ạ
mng ơi giúp mih với, mih cảm ơn nhiều ạ
 Giúp mình phiếu học tập 3 - câu 1,2 vs ạ
Giúp mình phiếu học tập 3 - câu 1,2 vs ạ 

