\(n_{Br_2}=\dfrac{8}{160}=0,05\left(mol\right)\\ C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ n_{C_2H_4}=n_{Br_2}=0,05\left(mol\right)\\ \Rightarrow V_{C_2H_4\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ \%V_{\dfrac{C_2H_4}{hh}}=\dfrac{1,12}{3,36}.100\approx33,33\%\\ \Rightarrow\%V_{\dfrac{CH_4}{hh}}=\dfrac{3,36-1,12}{3,36}.100\approx66,67\%\)
Bài 29: Anken
Đúng 3
Bình luận (0)
Các câu hỏi tương tự
Giúp em làm câu 21 đi ạ
Giúp e làm câu 7 đi ạ
Giúp em câu 4,5,6 ạ 
Mọi người giúp mình câu ba với ạ, cần gấp lắm ạ!! 😥😥😥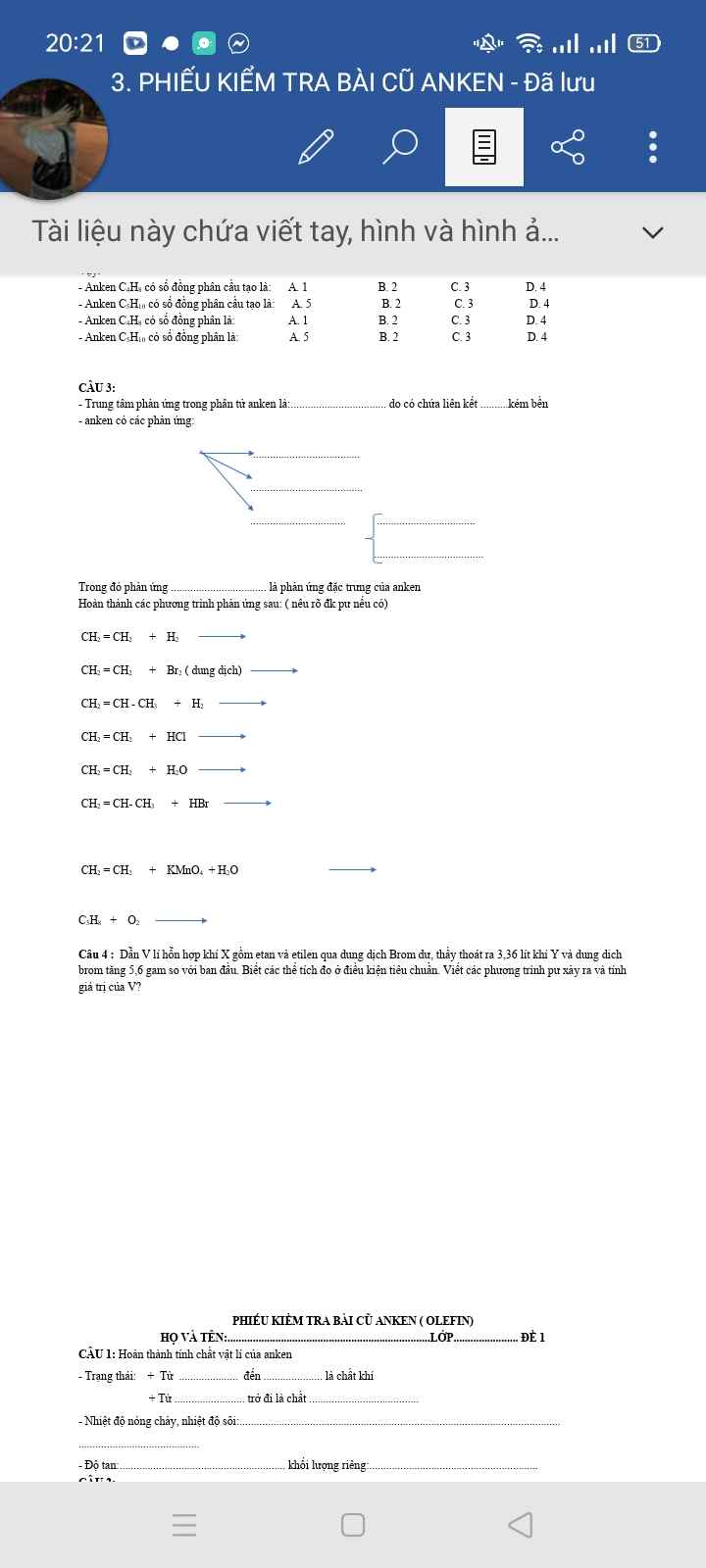
Giúp em giải với ạ 😭😭
Mọi người giải giúp em bài này với ạ! Em xin cảm ơn!
BT: Cho 3.36 gam một anken B tác dụng vừa đủ với dung dịch HBr thu được 12.96 gam ankyl bromua. Xác định công thức phân tử của B.
Mọi người giúp em giải bài này với ạ! Em xin cảm ơn!
BT: Hợp chất X có phần tẳm khối lượng C, H, O lần lượt bằng 54,54%; 9,10% và 36,36%. Khối lượng mol phân tử của X bằng 88. Xác định CTPT của X.
Cho hỗn hợp X gồm anken và hiđro có tỉ khối so với heli bằng 3,33. Cho X đi qua bột niken nung nóng đến khi phản ứng xảy ra hoàn toàn, thu được hỗn hợp Y có tỉ khối so với heli là 4. CTPT của X là.
MỌI NGƯỜI GIẢI THÍCH GIÚP EM TẠI SAO MY=16 THÌ KẾT LUẬN ĐƯỢC LÀ H2 DƯ Ạ
Ace trả lời hộ mình câu 4 5 với Em cảm ơn trước.!













