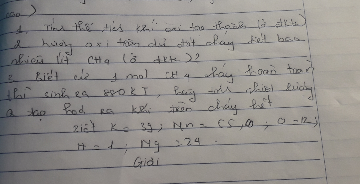\(3Fe+2O2-->Fe3O4\)
a)\(n_{Fe}=\frac{1,68}{56}=0,03\left(mol\right)\)
\(n_{Fe3O4}=\frac{1}{3}n_{Fe}=0,01\left(mol\right)\)
\(m_{Fe3O4}=0,01.232=2,32\left(g\right)\)
b) \(n_{O2}=\frac{2}{3}n_{Fe}=0,2\left(mol\right)\)
\(V_{O2}=0,2.22,4=4,48\left(l\right)\)
c) \(V_{kk}=5V_{O2}=4,48.5=22,4\left(l\right)\)