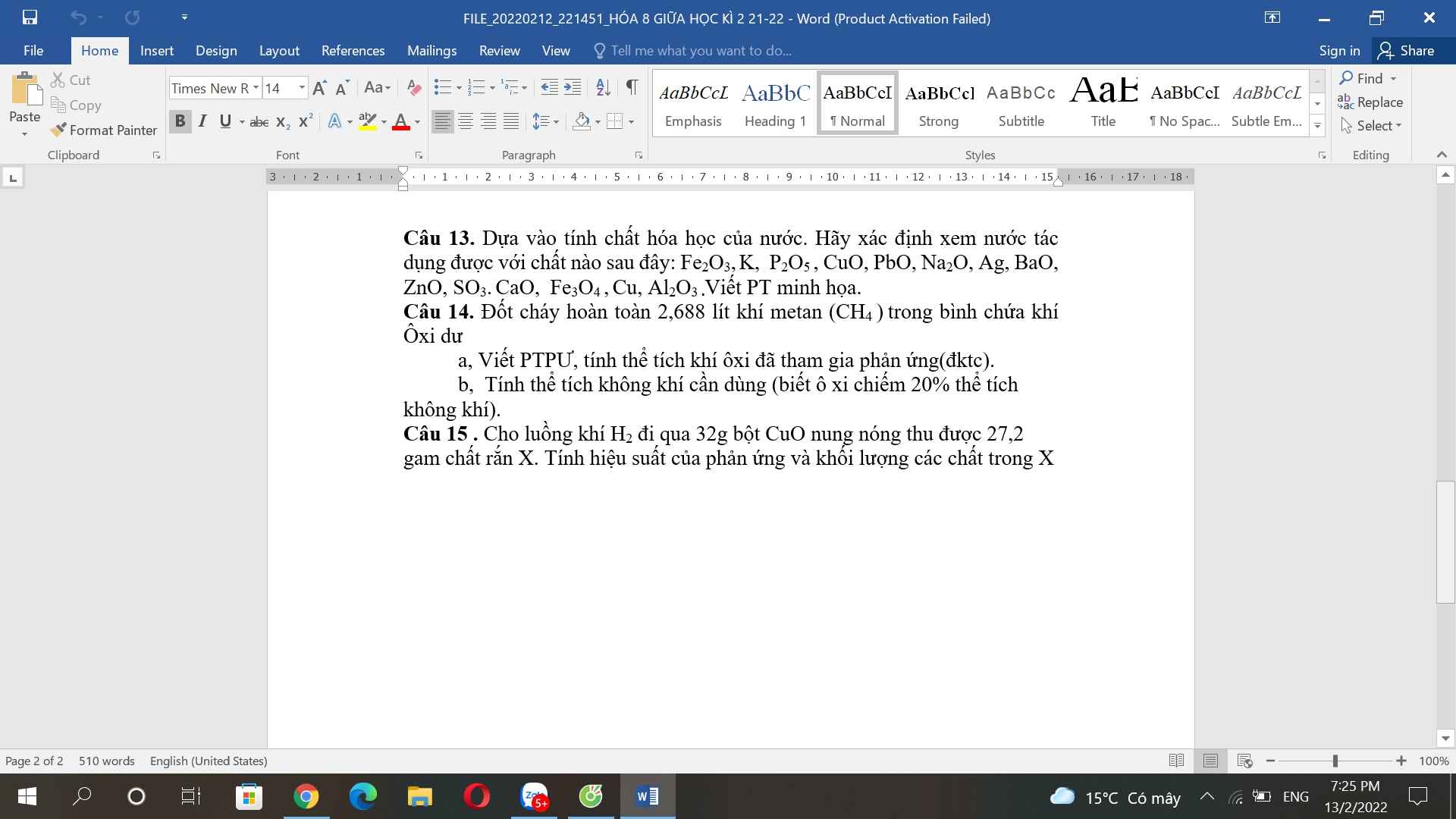
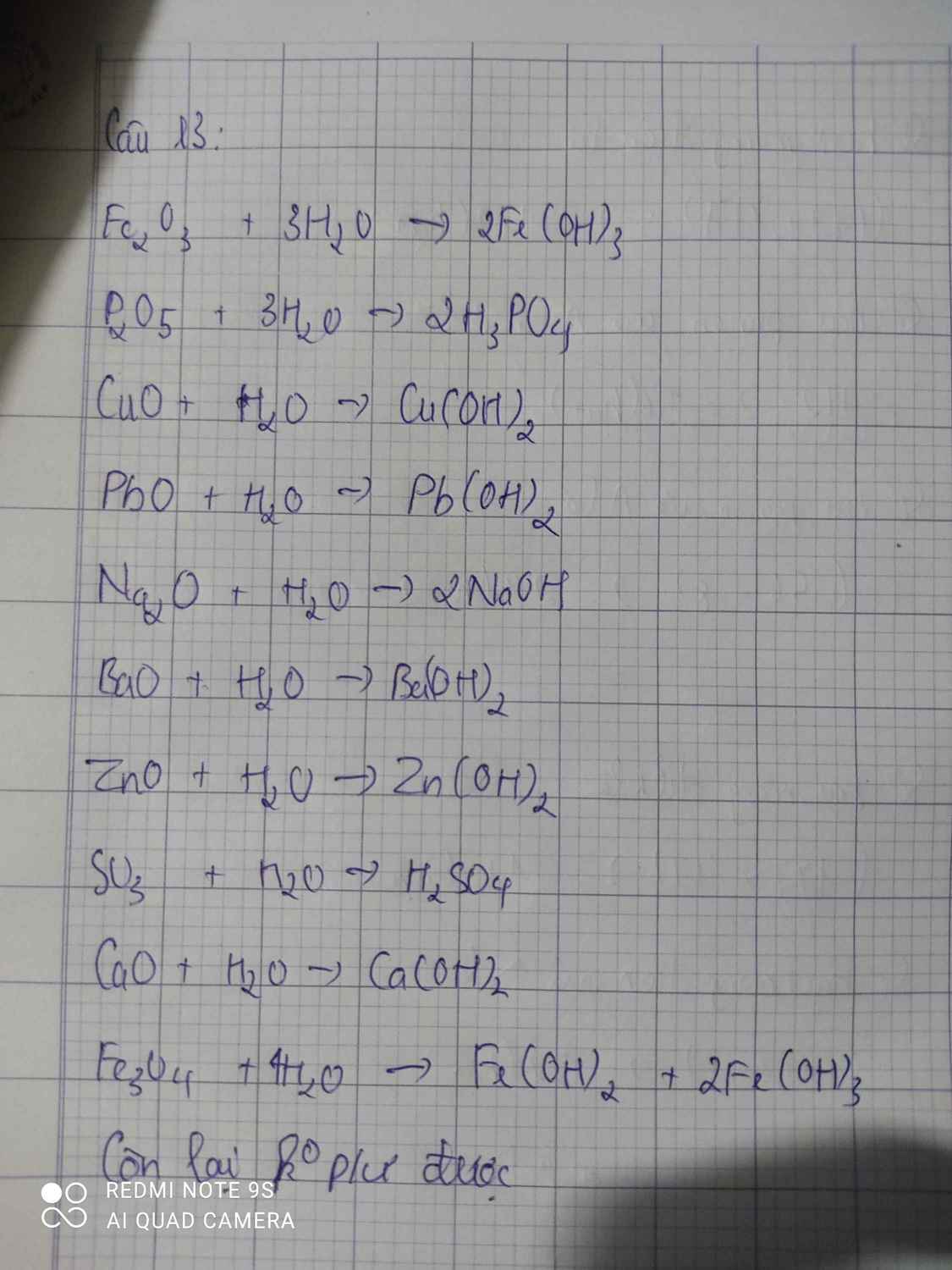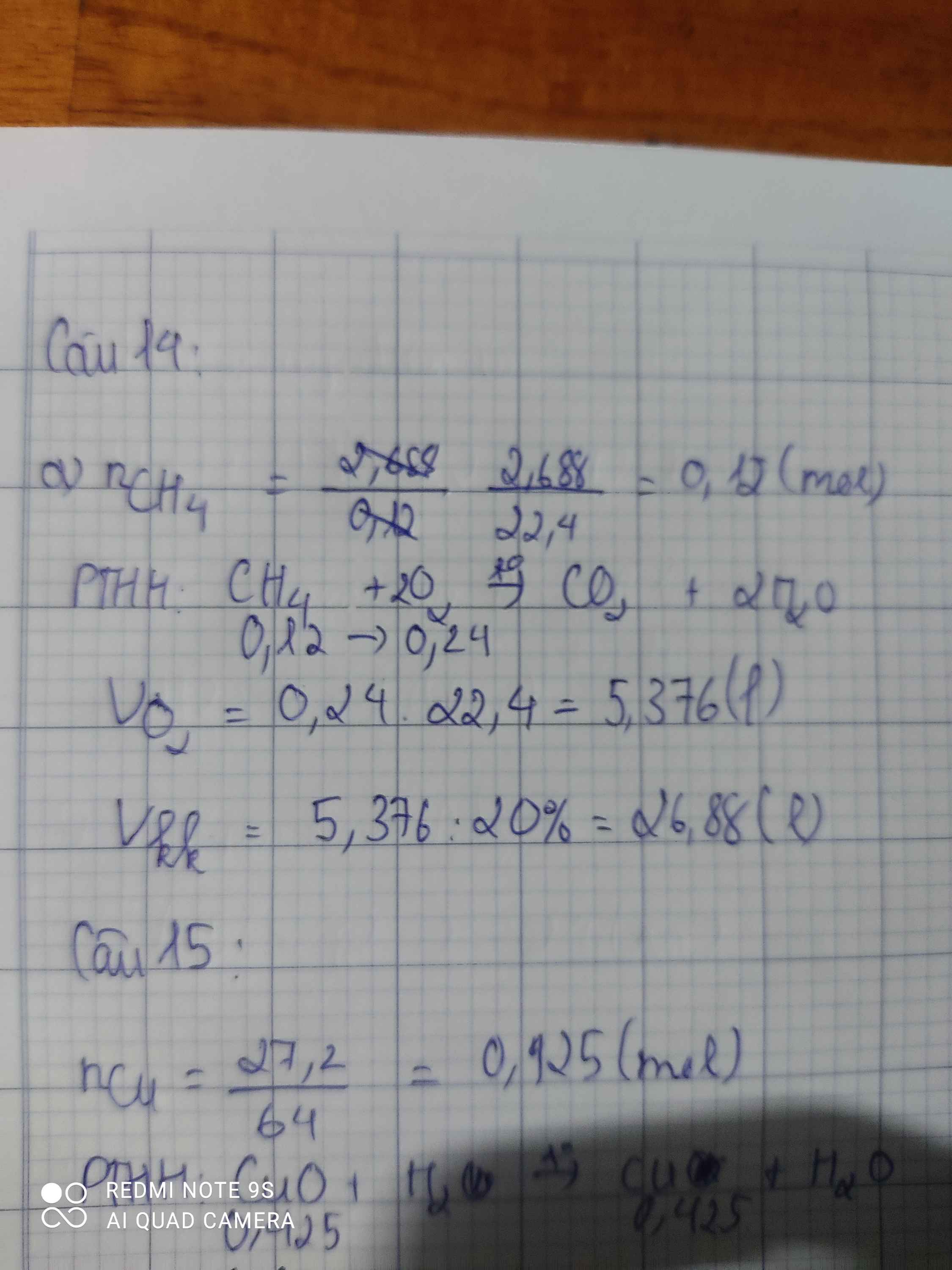Bài 15:
\(n_{CuO}=\dfrac{32}{80}=0,4\left(mol\right)\\ CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\\ TH1:CuO.p.ứ.hết\\ n_{Cu}=n_{CuO}=0,4\left(mol\right)\\ \Rightarrow m_X=m_{Cu}=0,4.64=25,6\left(g\right)\ne27,2\left(g\right)\\ \Rightarrow LoạiTH1\\ TH2:CuOdư\\ \Rightarrow X:CuO\left(dư\right),Cu\\ Đặt:n_{CuO\left(p.ứ\right)}=a\left(mol\right)\\ \Rightarrow m_X=64a+\left(32-80a\right)=27,2\\ \Leftrightarrow16a=4,8\\ \Leftrightarrow a=0,3\left(mol\right)\\ \Rightarrow H=\dfrac{0,3}{0,4}.100=75\%\\ X:\left\{{}\begin{matrix}0,1\left(mol\right)CuO\left(dư\right)\\0,3\left(mol\right)Cu\end{matrix}\right.\\ \Rightarrow m_{CuO\left(dư\right)}=0,1.80=8\left(g\right);m_{Cu}=64.0,3=19,2\left(g\right)\)