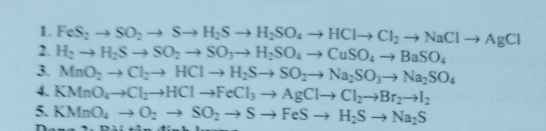Bài 2:
\(n_{CuSO_4}=n_{CuSO_4.2H_2O}=\dfrac{25}{196}\left(mol\right)\\C_{MddCuSO_4}=\dfrac{\dfrac{25}{196}}{0,2}\approx0,638\left(M\right) \)
Bài 3:
\(m_{CuSO_4}=\dfrac{160}{196}.25=\dfrac{1000}{49}\left(g\right)\\ C\%_{ddCuSO_4}=\dfrac{\dfrac{1000}{49}}{200}.100\approx10,204\%\)