\(m_H=\dfrac{1,59.63}{100}=1g\)
\(m_N=\dfrac{22,22.63}{100}\approx14g\)
\(m_O=\dfrac{76.19.63}{100}\approx48g\)
\(n_H=\dfrac{1}{1}=1mol\\ n_N=\dfrac{14}{14}=1mol\\ n_O=\dfrac{48}{16}=3mol\\ CTHH:HNO_3\)
\(m_N=\%N.M_M=22,22\%.63=14\left(g\right)\\ m_H=\%H.M_X=1,59\%.63=1\left(g\right)\\ m_O=m_X-m_H-m_N=63-14-1=48\left(g\right)\\ n_N=\dfrac{m}{M}=\dfrac{14}{14}=1\left(mol\right)\\ n_H=\dfrac{m}{M}=\dfrac{1}{1}=1\left(mol\right)\\ n_O=\dfrac{m}{M}=\dfrac{48}{16}=3\left(mol\right)\\ CTHH:HNO_3\)








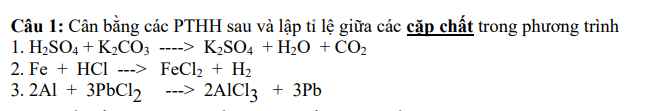
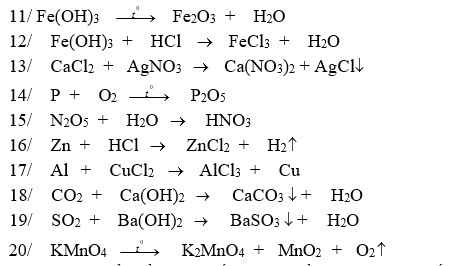
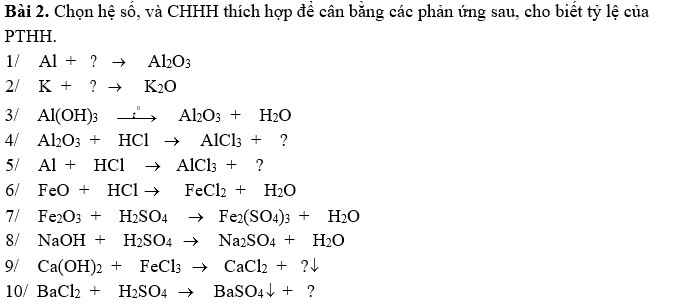







 giúp mình bài 7, bài 8 với ạ, cảm ơn nhiều
giúp mình bài 7, bài 8 với ạ, cảm ơn nhiều

