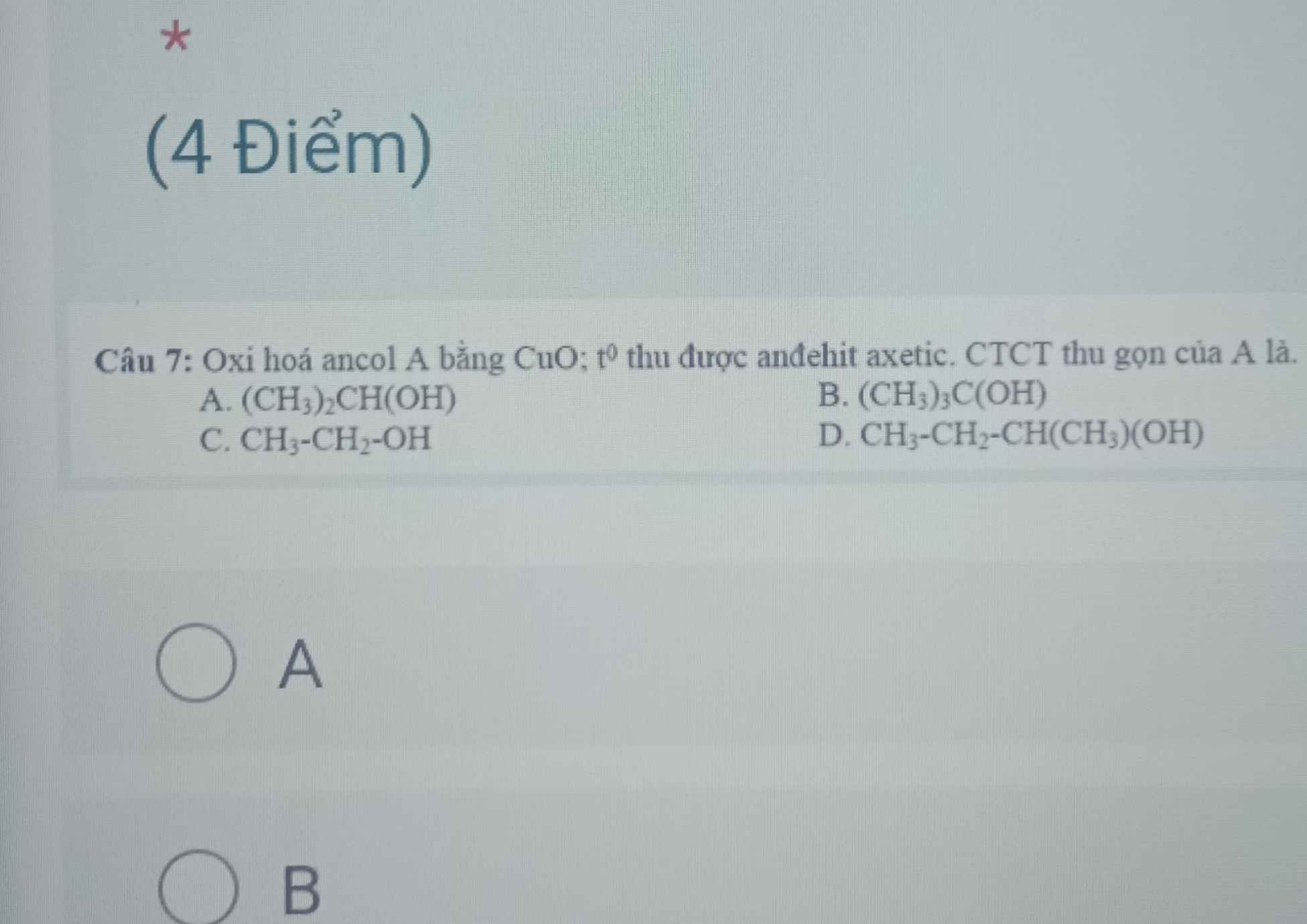
Câu 4 :
\(n_{H^+}=0.2\cdot0.5\cdot2+0.2\cdot0.25=0.25\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
\(0.25......0.25\)
\(n_{OH^-\left(dư\right)}=0.001V-0.25\left(mol\right)\)
\(C_{M_{OH^-\left(dư\right)}}=\dfrac{0.001V-0.25}{0.2+0.001V}\left(M\right)\)
\(pH=13\)
\(\Rightarrow log\left[OH^-\right]=13-14=-1\)
\(\Rightarrow log\left(\dfrac{0.001V-0.25}{0.2+0.001V}\right)=-1\)
\(\Rightarrow V=300\)
Câu 5 :
\(pH=1\Rightarrow\left[H^+\right]=0.1\)
\(pH=13\Rightarrow\left[OH^-\right]=0.1\)
\(n_{H^+}=0.1V_1\left(mol\right)\)
\(n_{OH^-}=0.1V_2\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
\(0.1V_1...0.1V_1\)
\(n_{OH^-\left(dư\right)}=0.1V_2-0.1V_1\left(mol\right)\)
\(\left[OH^-\right]\left(dư\right)=\dfrac{0.1V_2-0.1V_1}{V_1+V_2}\left(M\right)\)
\(pH=14+log\left[OH^-\right]=12\)
\(\Rightarrow\left[OH^-\right]=0.01\)
\(\Rightarrow\dfrac{0.1V_2-0.1V_1}{V_1+V_2}=0.01\)
\(\Leftrightarrow V_2-V_1-0.1V_1-0.1V_2=0\)
\(\Leftrightarrow0.9V_2-1.1V_1=0\)
\(\Leftrightarrow\dfrac{V_1}{V_2}=\dfrac{0.9}{1.1}=\dfrac{9}{11}\)