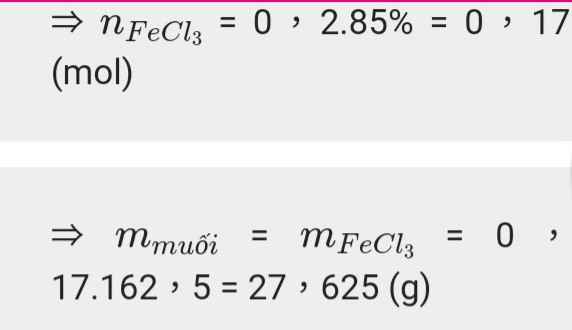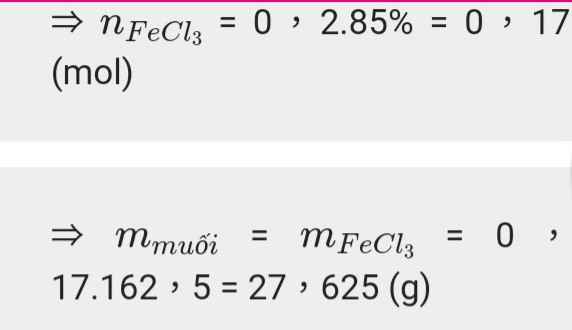a) \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH: 2Fe + 3Cl2 --to--> 2FeCl3
_____0,2---->0,3---------->0,2
=> VCl2 = 0,3.22,4 = 6,72(l)
b) PTHH: FeCl3 + 3NaOH --> Fe(OH)3\(\downarrow\) + 3NaCl
________0,2---------------------->0,2
=> mFe(OH)3 = 0,2.107=21,4(g)