\(n_{SO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right);n_{NaOH}=1.0,15=0,15\left(mol\right)\\ Vì:1< \dfrac{n_{NaOH}}{n_{SO_2}}=\dfrac{0,15}{0,1}=1,5< 2\\ \Rightarrow Sp:Na_2SO_3,NaHSO_3\\ PTHH:2NaOH+SO_2\rightarrow Na_2SO_3+H_2O\left(1\right)\\ NaOH+SO_2\rightarrow NaHSO_3\left(2\right)\\ Đặt:n_{NaOH\left(1\right)}=a\left(mol\right);n_{NaOH\left(2\right)}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}a+b=0,15\\0,5a+b=0,1\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,05\end{matrix}\right.\\ \Rightarrow m_{muối}=0,5.a.126+b.104=11,5\left(g\right)\)
Bài 32: Hiđro sunfua-Lưu huỳnh Đioxit - Lưu huỳnh Trioxit
Đúng 3
Bình luận (0)
Các câu hỏi tương tự
Giúp em với ạ. Em cảm ơn nhiều
Bài 1:Cho 3,36 lít SO2 phản ứng với V ml dung dịch NaOH 1M.Tìm V để khối lượng muối sau phản ứng là lớn nhất.
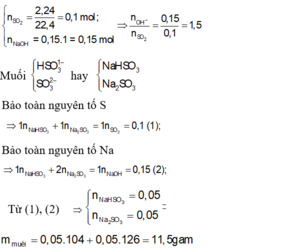
Bài 2: cho 2,24 l So2 phản ứng với V ml dung dịch Naoh 1M . Tìm V để khối lượng muối thu được sau phản ứng là nhỏ nhất
Hấp thụ hoàn toàn 1.12 lít khí so2 ( đktc) vào 50.0ml dd naoh 1M thu được dd X. dd X chứa các chất tan
Bài 1: Cho 3,36 lít SO2 đktc hấp thụ vào 500ml dung dịch NaOH 0,1M. Tính khối lượng muối thu được
Câu 2.Hấp thụ hoàn toàn 12,8 gam SO2 vào 250 ml dung dịch NaOH 1M.
a) Viết các phương trình hóa học của phản ứng xảy ra. b) Tính khối lượng muối tạo thành sau phản ứng.Hấp thụ hoàn toàn 2,24 lít khí SO2 (đktc) vào 200ml dd NaOH 1,5M
a, Viết pthh của các phản ứng có thể xảy ra
b, Tính khối lượng muối tạo thành sau phản ứng
Câu1:tính nồng độ các chất trong dd thu đc : dẫn 5.6 lít khí SO2 vào 400ml dd NaOH 1.5M
Câu2:tính khối lượng muối thu đc sau pứng : Cho 25.6 gam khi SO2 hấp thụ hoàn toàn vào 500ml dd NaOH 1M
Câu 3 Tính nồng độ mol của dd naoh đã dùng : hấp thụ hoàn toàn 3.36 lít khí so2 vào 500ml dd NaOH thu đc 16.7 gam hỗn hợp 2 muối khan
Bài 1: Cho 3,36 lít SO2 đktc hấp thụ vào 500ml dung dịch NaOH 0,1M. Tính khối lượng muối thu được
Bài 2: Cho 2,24 lít SO2 đktc hấp thụ hết vào 200ml dung dịch KOH 0,1M. Tính khối lượng muối thu được
Bài 3: Cho 3,36 lít H2S đktc hấp thụ vào 500ml dung dịch NaOH 0,1M. Tính khối lượng muối thu được
Hấp thụ hoàn toàn 12,8g SO2 vào 250 ml dung dịch NaOH 1M.
a) Viết các phương trình hóa học của các phản ứng có thể xảy ra.
b) Tính khối lượng muối tạo thành sau phản ứng.
Bài 1:
Sục 6,72 l SO2 ở đktc vào 1 l hỗn hợp gồm NaOH 0,2M và KOH 0,2M. Xác định khối lượng muối thu được sau phản ứng.
Bài 2:
Dẫn 2,24 l khí SO2 vào 100 ml hỗn hợp dd gồm KOH 1M và Ba(OH)2 0,75M, sau khi khí bị hấp thuj hoàn toàn thấy tạo ra m g kết tủa. Tính m