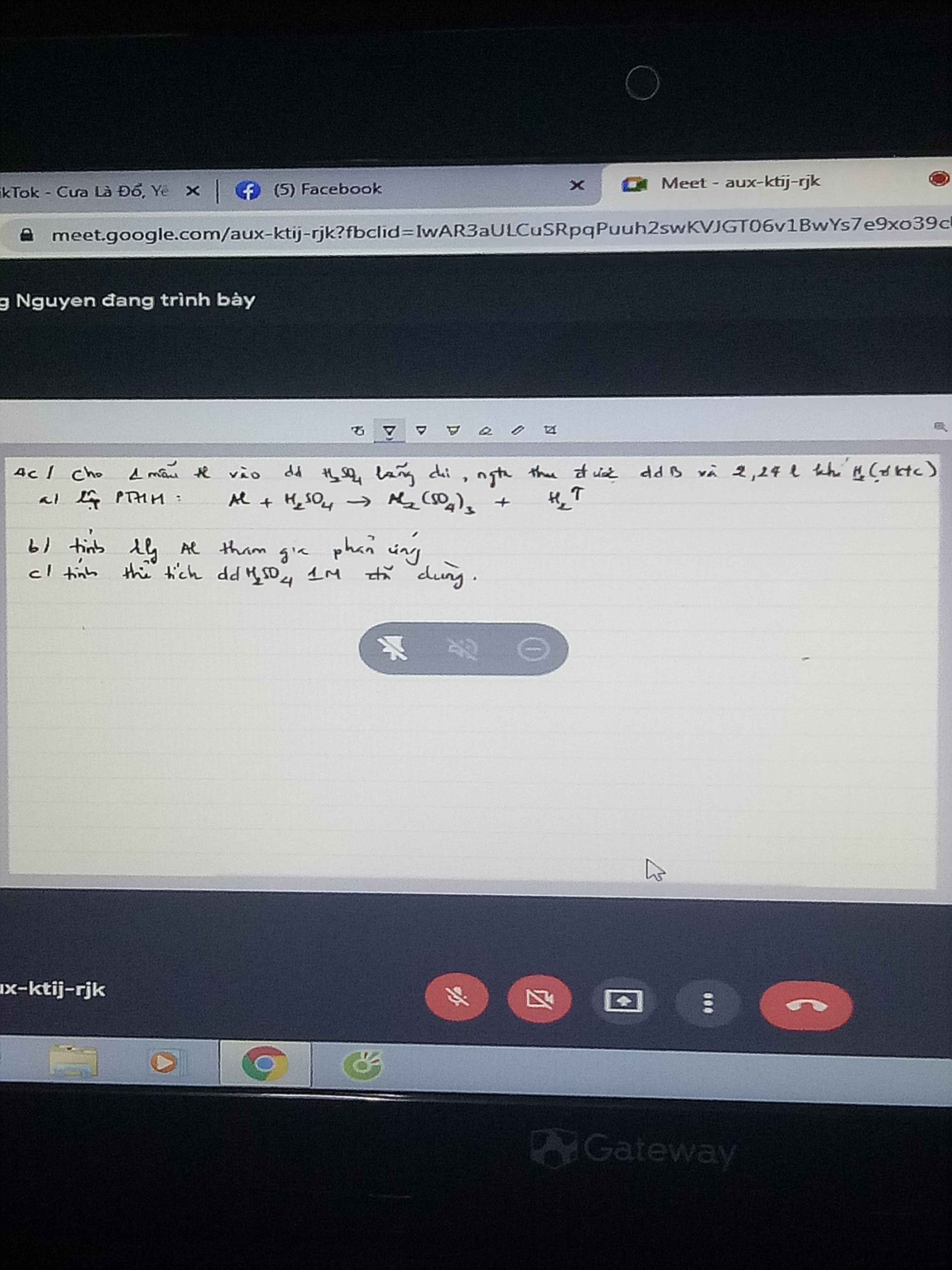
$a)PTHH:2Al+3H_2SO_4\to Al_2(SO_4)_3+3H_2\uparrow$
$b)n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)$
Theo PT: $n_{Al}=\dfrac{2}{3}n_{H_2}=\dfrac{1}{15}(mol)$
$\Rightarrow m_{Al}=\dfrac{1}{15}.27=1,8(g)$
$c)$ Theo PT: $n_{H_2SO_4}=n_{H_2}=0,1(mol)$
$\Rightarrow V_{dd_{H_2SO_4}}=\dfrac{0,1}{1}=0,1(l)=100(ml)$