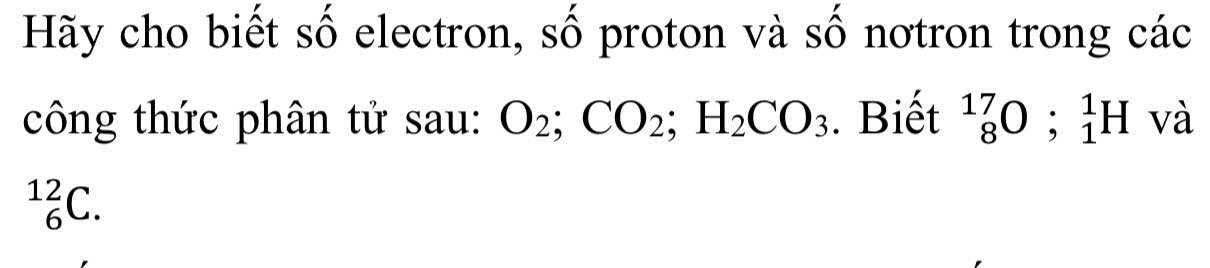\(O_2\\ P=E=P_O.2=8.2=16\left(hạt\right)\\ N=\left(A_O-P_O\right).2=\left(17-8\right).2=18\left(hạt\right)\\ CO_2\\ P_{CO_2}=E_{CO_2}=P_C+2.P_O=6+2.8=22\left(hạt\right)\\ N_{CO_2}=N_C+2.N_O=\left(A_C-P_C\right)+2.\left(A_O-P_O\right)\\ =\left(12-6\right)+2.\left(17-8\right)=24\left(hạt\right)\\ H_2CO_3\\ E_{H_2CO_3}=P_{H_2CO_3}=2.P_H+P_C+3.P_O=2.1+6+8.3=32\left(hạt\right)\\ N_{H_2CO_3}=\left(A_H-P_H\right).2+\left(A_C-P_C\right)+3.\left(A_O-P_O\right)\\ =0.2+6+3.9=33\left(hạt\right)\)
Bài 2. Hạt nhân nguyên tử. Nguyên tố hóa học. Đồng vị
Đúng 1
Bình luận (1)
Các câu hỏi tương tự
Số khối của hạt nhân nguyên tử canxi bằng 40 hạt nhân có 20 nowtron xác định Z,E của Cãni
Oxi hóa hoàn toàn 18,4 gam hỗn hợp gồm Zn và Al cần vừa đúng 5,6 lít Oxi (đktc). Tính thành phần % khối lượng mỗi kim loại trong hh.
Hỗn hợp A gồm 2 chất kế tiếp nhau trong dãy đồng đẳng etilen, cho 3,36 lít hỗn hợp khí trên phản ứng hoàn toàn với Br2 trong ccl4 thì khối lượng trong bình chứa br tăng 7,7g,
Hãy xác định thành phần % về thể tích của hỗn hợp A.
Bài này nx nha
nguyên tử x có 11 hạt mang điện tích âm tổng số hạt cơ bản trong nguyên tử x là 34 viết kí hiệu nguyên tử x
Hòa tan hoàn toàn m gam Al cần dùng 600ml dung dịch h2so4 0,5M(loãng) thu đc dung dịch Y (chứa m gam muối)và 4,48lits h2(đktc).tính khối lượng mỗi kim loại trong x và tính m
Giải giúp em với ạ.Em cảm ơn
Một nguyên tử kim loại R có tổng số hạt là 58 .Xác định số p,n,e
Phân lớp electron ngoài cùng của hai nguyên tử A và B lần lượt là 3p và 4s. Tổng số electron của 2 phân lớp bằng 5 và hiệu số electron của chúng bằng 3. Tổng số hạt mang điện trong 2 nguyên tử A và B là *
Cấu hình e phân lớp ngoài cùng của nguyên tử của hai nguyên tố A và B lần lượt là 3sx và 3p5.Biết rằng phân lớp 3s của hai nguyên tử A và B hơn kém nhau 1 electron.Hai nguyên tố A,B là gì?
Ai giúp e với nguyên tử Al có 13 hạt proton và 14 hạt nơtron. Điện tích hạt nhân của Al là bao nhiêu ? Tính số khối của nguyên tử Al?