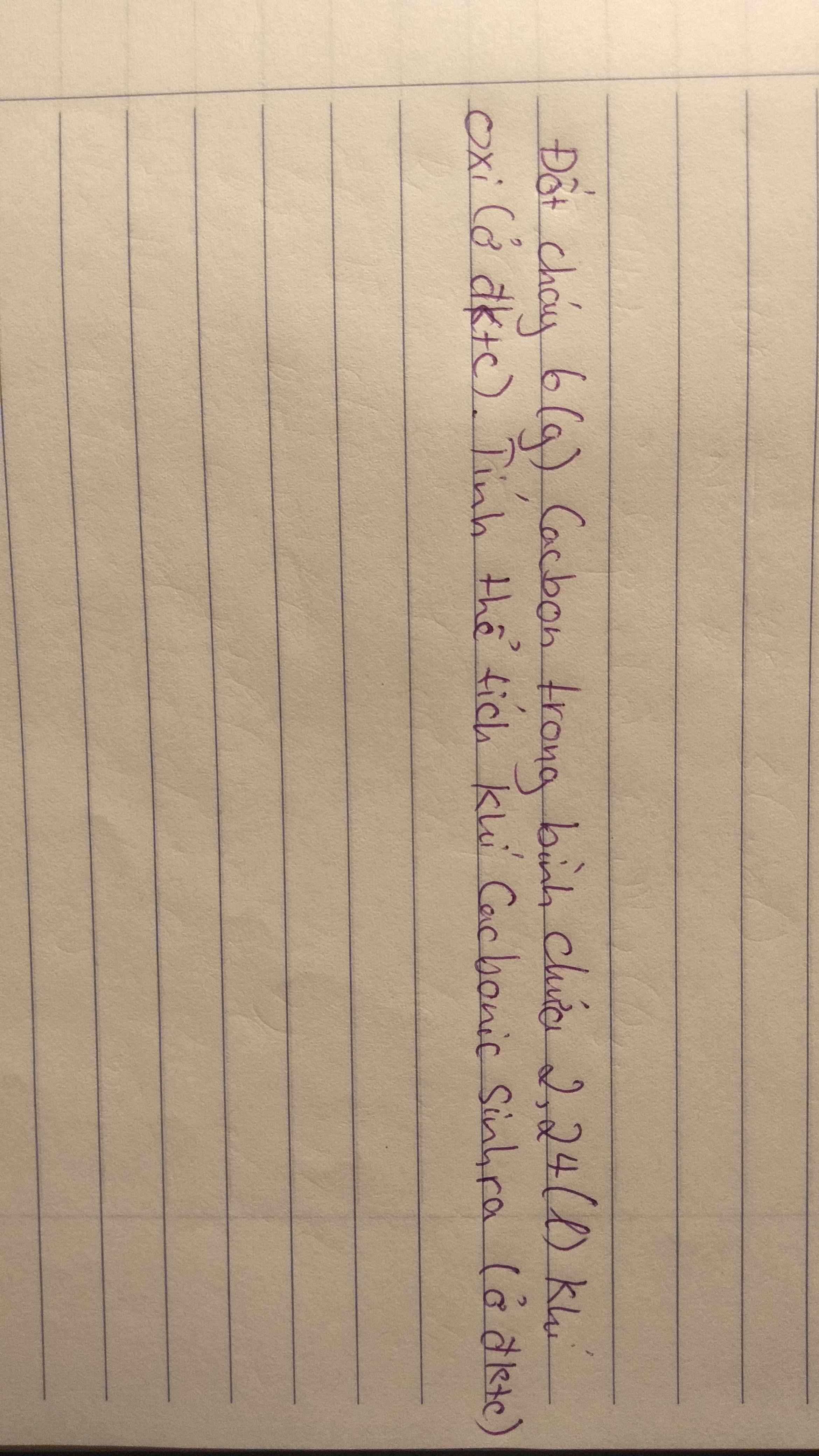nC = \(\dfrac{6}{12}=0,5\left(mol\right)\)
\(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: C + O2 ---to---> CO2.
Ta thấy: \(\dfrac{0,5}{1}>\dfrac{0,1}{1}\)
=> Cacbon dư
Theo PT: \(n_{CO_2}=n_{O_2}=0,1\left(mol\right)\)
=> \(V_{CO_2}=0,1.22,4=2,24\left(lít\right)\)