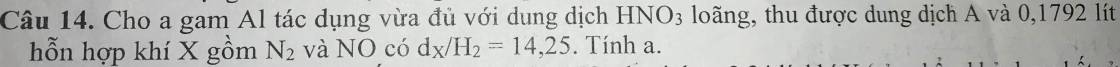\(10Al+36HNO_{3\left(l\right)}\rightarrow10Al\left(NO_3\right)_3+3N_2\uparrow+18H_2O\)
\(Al+4HNO_{3\left(l\right)}\rightarrow Al\left(NO_3\right)_3+NO\uparrow+2H_2O\)
\(n_{hhk}=\dfrac{0,1792}{22,4}=8.10^{-3}\left(mol\right)\)
\(\Rightarrow n_{N_2}+n_{NO}=8.10^{-3}\left(1\right)\)
\(d_{\dfrac{X}{H_2}}=14,25\)
\(\Rightarrow M_X=28,5\) \((g/mol)\)
\(\Leftrightarrow\dfrac{28.n_{N_2}+30.n_{NO}}{n_{N_2}+n_{NO}}=28,5\)
\(\Leftrightarrow\dfrac{28.n_{N_2}+30.n_{NO}}{8.10^{-3}}=28,5\)
\(\Leftrightarrow28.n_{N_2}+30.n_{NO}=0,228\left(2\right)\)
\(\left(1\right);\left(2\right)\Rightarrow\left\{{}\begin{matrix}n_{N_2}=6.10^{-3}\\n_{NO}=2.10^{-3}\end{matrix}\right.\) ( mol )
\(n_{Al}=\dfrac{10}{3}.6.10^{-3}+2.10^{-3}=22.10^{-3}\left(mol\right)\)
\(m_{Al}=22.10^{-3}.27=0,594\left(g\right)\)