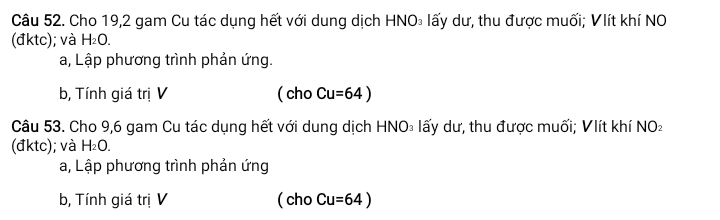
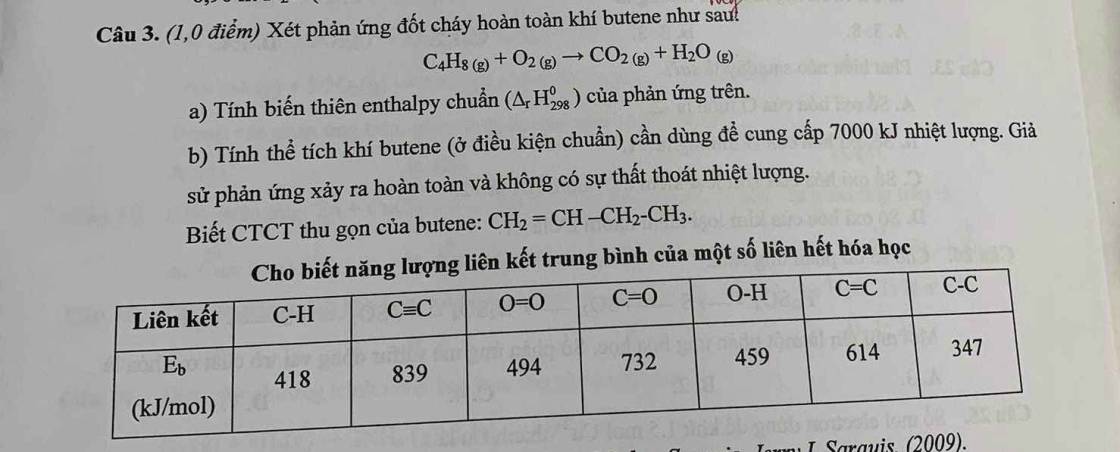
Câu 52:
\(n_{Cu}=\dfrac{19,2}{64}=0,3(mol)\\ a,3Cu+8HNO_3\to 3Cu(NO_3)_2+2NO\uparrow+4H_2O\\ b,n_{NO}=0,2(mol)\\ \Rightarrow V_{NO}=0,2.22,4=4,48(l)\)
Câu 53:
\(n_{Cu}=\dfrac{9,6}{64}=0,15(mol)\\ a,Cu+4HNO_3\to Cu(NO_3)_2+2NO_2\uparrow+2H_2O\\ b,n_{NO_2}=0,3(mol)\\ \Rightarrow V_{NO_2}=0,3.22,4=6,72(l)\)
Đúng 1
Bình luận (0)