Giúp e vs ạ


Những câu hỏi liên quan
Giúp e vs ạ😢🐰
a,NTK của Y là:1,5.16=24 (g/mol)
NTK của X là: \(\dfrac{1}{2}.24=12\left(g/mol\right)\)
b,KHHH của X là C
của Y là Mg
của Z là O
Đúng 2
Bình luận (0)
Giúp vs mk cần gấp 🐰🐰🐰
Đề 1:
1. HCST: “Chiếc lược ngà” viết năm 1966, tại chiến trường Nam Bộ trong thời kì kháng chiến chống Mĩ và được đưa vào tập truyện cùng tên
2. ND: Nỗi buồn của anh Sáu khi con gái không nhận ra mình
3. TP tình thái: Với lòng mong nhớ, chắc...
Khởi ngữ: Với lòng mong nhớ
Phép thế: con bé-> nó
Đúng 1
Bình luận (2)
mn giúp e vs ạ 😢
Tìm x:
\(\left|x+1\right|+\left|2x+1\right|=5\)
Giúp mk vs ạ😢😢
XVI Rewrite the following sentences based on the given words below.
5. She has lived in New York since 2005 => (started)
Giúp e vs ạ😢😢
bạn muốn dịch hay thế nào
Chuyển sang thì quá khứ đơn đúng khô?
-She lived in New York was in 2005
ks nhé!Học tốt!:))
mk làm vậy ko bt cs đunk ko
she started lived in new york since 2005
theo đề bài bn thì chắc vậy đúng thì lick ko thì thui
Xem thêm câu trả lời
giúp mk vs ạ 😢
A song was sung by her.
Was the letter sent by him?
The bus was stopped.
My car was stolen by a thief.
He was not let go.
Đúng 1
Bình luận (0)
The prize wasn't won by her.
Their beds weren't made.
They were not told by me.
Were they told by you?
I was hit by him.
Đúng 1
Bình luận (0)
The letter was lost by my mother yesterday.
They key was found by her at home.
Mistakes were made.
That woman was loved by Peter 3 years ago.
The rooms were cleaned.
Đúng 1
Bình luận (0)
Xem thêm câu trả lời
giúp mk vs ạ 😢
Câu 1 :
Gọi $m_{dd} = a(gam)$
$n_{NaOH} = \dfrac{a.20\%}{40} = 0,005a(mol)$
$n_{Ba(OH)_2} = \dfrac{a.8,55\%}{171} = 0,0005a(mol)$
$n_{HNO_3} = \dfrac{224}{1,1.1000}.4,5 = 0,9(mol)$
$NaOH + HNO_3 \to NaNO_3 + H_2O$
$Ba(OH)_2 + 2HNO_3 \to Ba(NO_3)_2 + 2H_2O$
Theo PTHH :
$0,005a + 0,0005a.2 = 0,9 \Rightarrow a = 150(gam)$
Đúng 2
Bình luận (0)
Câu 2 :
Gọi thể tích dung dịch cần tìm là V(lít)
$n_{H_2SO_4} = 1,1V(mol) ; n_{HCl} = 1,98V(mol)$
$\Rightarrow n_H = 1,1V.2 + 1,98V = 4,18V(mol)$
$n_{NaOH} = 0,19.3 = 0,57(mol) ; n_{Ba(OH)_2} = 0,19.4 = 0,76(mol)$
$\Rightarrow n_{OH} = 0,57 + 0,76.2 = 2,09(mol)$
$H + OH \to H_2O$
$\Rightarrow 4,18V = 2,09 \Rightarrow V = 0,5(lít)$
Đúng 1
Bình luận (0)
Câu 3 :
$n_{ZnO} = \dfrac{48,6}{81} = 0,6(mol) ; n_{HNO_3} = \dfrac{800.6,3\%}{63}= 0,8(mol)$
ZnO + 2HNO3 → Zn(NO3)2 + H2O
0,4.......0,8...............0,4........................(mol)
Sau phản ứng :
$m_{dd} = 0,4.81 +800 = 832,4(gam)$
$C\%_{Zn(NO_3)_2} = \dfrac{0,4.189}{832,4}.100\% = 9,1\%$
Đúng 2
Bình luận (0)
Xem thêm câu trả lời
Mn giúp em vs ạ😢
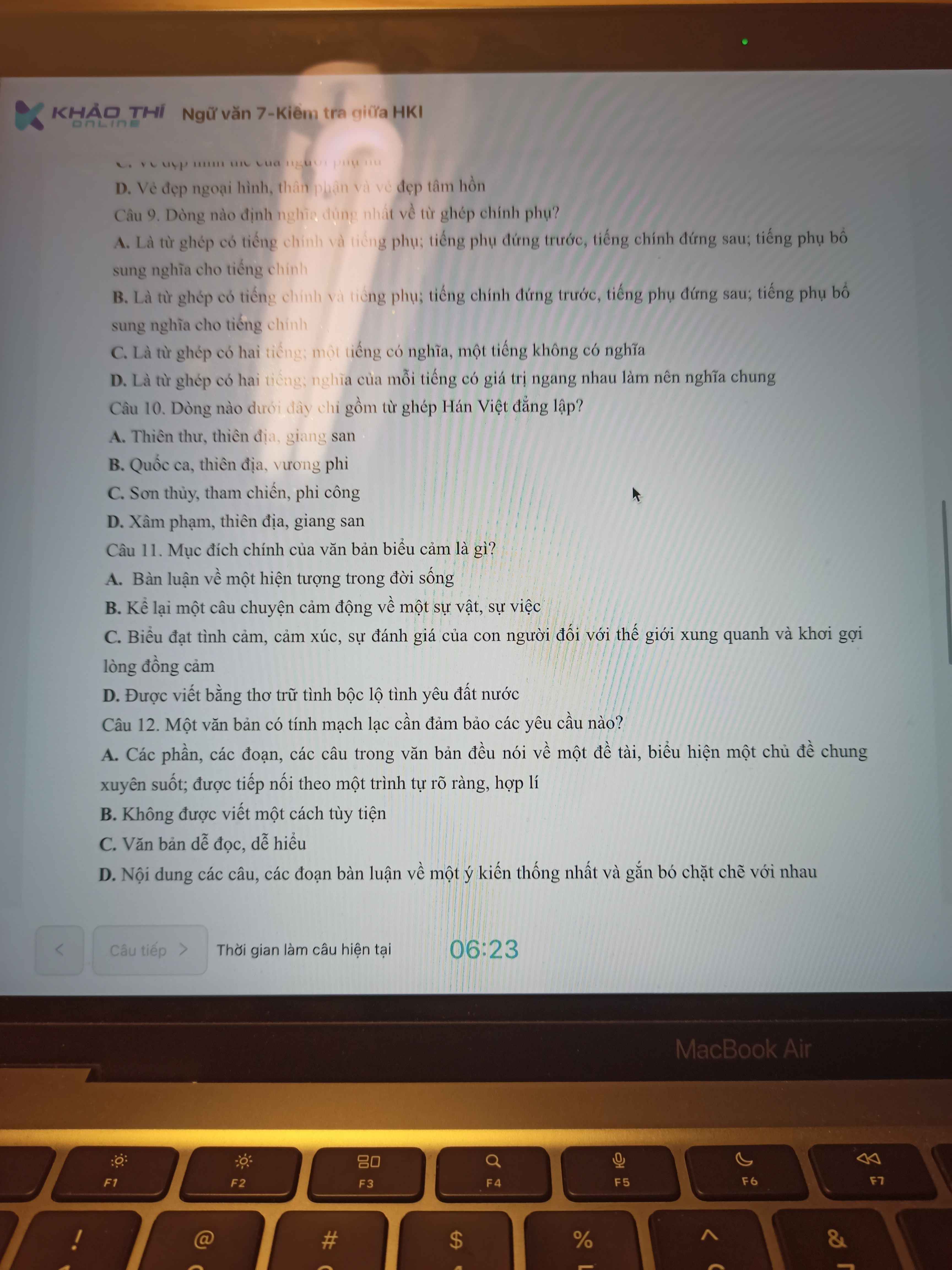
1 A
2 C
3 C
4 D
5 B
6 A
7 B
8 D
9 C
10 C
11 B
12 D
13 B
14 C
15 B
Đúng 1
Bình luận (0)
Mn giúp em vs ạ 😢
Giúp mik vs ạ🥺😢🤧
1. She usually wears a red uniform.
2. There was a concert yesterday.
Đúng 3
Bình luận (0)
1. She usually wears a red uniform
2. There was a concert yesterday
Đúng 2
Bình luận (0)
1. She usually wears a red uniform.
2. There was a concert yesterday.
Đúng 0
Bình luận (0)





























