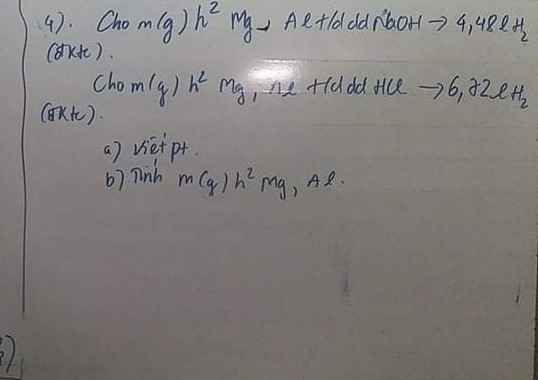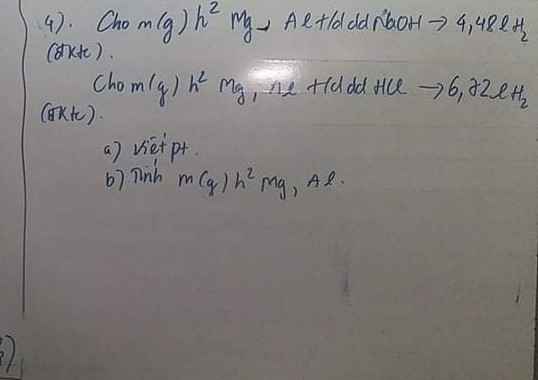Câu 1 :
Gọi $m_{dd} = a(gam)$
$n_{NaOH} = \dfrac{a.20\%}{40} = 0,005a(mol)$
$n_{Ba(OH)_2} = \dfrac{a.8,55\%}{171} = 0,0005a(mol)$
$n_{HNO_3} = \dfrac{224}{1,1.1000}.4,5 = 0,9(mol)$
$NaOH + HNO_3 \to NaNO_3 + H_2O$
$Ba(OH)_2 + 2HNO_3 \to Ba(NO_3)_2 + 2H_2O$
Theo PTHH :
$0,005a + 0,0005a.2 = 0,9 \Rightarrow a = 150(gam)$
Câu 2 :
Gọi thể tích dung dịch cần tìm là V(lít)
$n_{H_2SO_4} = 1,1V(mol) ; n_{HCl} = 1,98V(mol)$
$\Rightarrow n_H = 1,1V.2 + 1,98V = 4,18V(mol)$
$n_{NaOH} = 0,19.3 = 0,57(mol) ; n_{Ba(OH)_2} = 0,19.4 = 0,76(mol)$
$\Rightarrow n_{OH} = 0,57 + 0,76.2 = 2,09(mol)$
$H + OH \to H_2O$
$\Rightarrow 4,18V = 2,09 \Rightarrow V = 0,5(lít)$
Câu 3 :
$n_{ZnO} = \dfrac{48,6}{81} = 0,6(mol) ; n_{HNO_3} = \dfrac{800.6,3\%}{63}= 0,8(mol)$
ZnO + 2HNO3 → Zn(NO3)2 + H2O
0,4.......0,8...............0,4........................(mol)
Sau phản ứng :
$m_{dd} = 0,4.81 +800 = 832,4(gam)$
$C\%_{Zn(NO_3)_2} = \dfrac{0,4.189}{832,4}.100\% = 9,1\%$
Bài 4 :
TH1 :
$HNO_3 + KOH \to KNO_3 + H_2O$
$n_{KOH} = n_{HNO_3} \Rightarrow 0,02a = 0,06b(1)$
TH2 :
$CuO + 2HNO_3 \to Cu(NO_3)_2 + H_2O$
$KOH + HNO_3 \to KNO_3 + H_2O$
$n_{HNO_3} = 2n_{CuO} + n_{KOH} = 2.\dfrac{2}{80} + 0,01b = 0,02a(2)$
Từ (1)(2) suy ra a = 3 ; b = 1