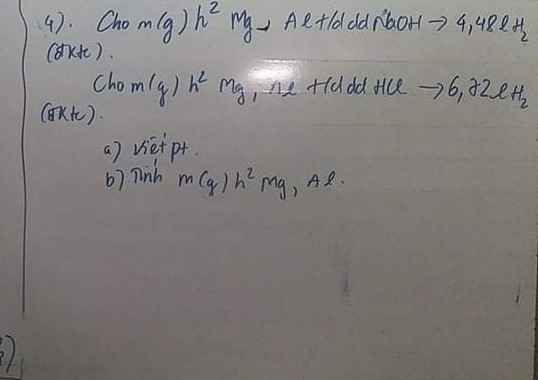Câu 3:
\(a,n_{Fe}=x(mol);n_{Zn}=y(mol)\\ \Rightarrow 56x+65y=18,6(1)\\ n_{H_2}=\dfrac{6,72}{22,4}=0,3(mol)\\ PTHH:Zn+H_2SO_4\to ZnSO_4+H_2\\ Fe+H_2SO_4\to FeSO_4+H_2\\ \Rightarrow x+y=0,3(2)\\ (1)(2)\Rightarrow x=0,1;y=0,2\\ \Rightarrow \%_{Fe}=\dfrac{0,1.56}{18,6}.100\%=30,11\%\\ \Rightarrow \%_{Zn}=100\%-30,11\%=69,89\%\)
\(b,\Sigma n_{H_2SO_4}=x+y=0,3(mol)\\ \Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,3}{0,1}=3M\\ c,n_{ZnSO_4}=0,2(mol);n_{FeSO_4}=0,1(mol)\\ \Rightarrow m_{ZnSO_4}=0,2.161=32,2(g)\\ m_{FeSO_4}=0,1.152=15,2(g)\)