Mn giúp em vs ạ 😢

Những câu hỏi liên quan
Mn giúp em vs ạ😢
1 A
2 C
3 C
4 D
5 B
6 A
7 B
8 D
9 C
10 C
11 B
12 D
13 B
14 C
15 B
Đúng 1
Bình luận (0)
mn giúp e vs ạ 😢
Tìm x:
\(\left|x+1\right|+\left|2x+1\right|=5\)
giúp em vs ạ những câu nào mờ quá thì thôi ạ 😢



Giúp mk vs ạ😢😢
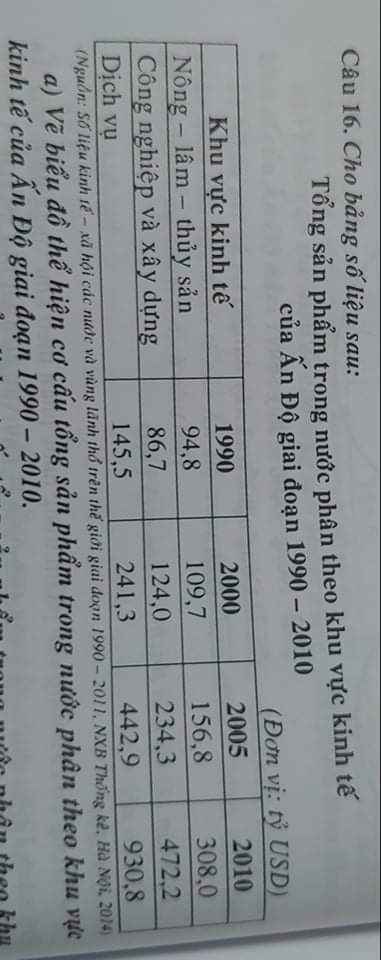
Mọi người giúp em với ạ, em cần gấp lắm ạ 😢😢
Giúp e bài n với mn ơi e c.ơn ạ mong mn giúp e😢
\(a,B=4\sqrt{x+1}-3\sqrt{x+1}+\sqrt{x+1}+2\sqrt{x+1}=4\sqrt{x+1}\\ b,B=8\Leftrightarrow4\sqrt{x+1}=8\\ \Leftrightarrow\sqrt{x+1}=2\\ \Leftrightarrow x+1=4\\ \Leftrightarrow x=3\left(tm\right)\)
Đúng 0
Bình luận (0)
Mọi người giúp em với ạ. Em đang cần gấp gấp lắm ạ 😢😢
MgO: Mg có điện hóa trị 2+, O có điện hóa trị 2-
FeF3: Fe có điện hóa trị 3+, F có điện hóa trị 1-
BaCl2: Ba có điện hóa trị 2+, Cl có điện hóa trị 1-
Ca3N2: Ca có điện hóa trị 2+, N có điện hóa trị 3-
Đúng 2
Bình luận (0)
giúp mk vs ạ 😢
A song was sung by her.
Was the letter sent by him?
The bus was stopped.
My car was stolen by a thief.
He was not let go.
Đúng 1
Bình luận (0)
The prize wasn't won by her.
Their beds weren't made.
They were not told by me.
Were they told by you?
I was hit by him.
Đúng 1
Bình luận (0)
The letter was lost by my mother yesterday.
They key was found by her at home.
Mistakes were made.
That woman was loved by Peter 3 years ago.
The rooms were cleaned.
Đúng 1
Bình luận (0)
Xem thêm câu trả lời
giúp mk vs ạ 😢
Câu 1 :
Gọi $m_{dd} = a(gam)$
$n_{NaOH} = \dfrac{a.20\%}{40} = 0,005a(mol)$
$n_{Ba(OH)_2} = \dfrac{a.8,55\%}{171} = 0,0005a(mol)$
$n_{HNO_3} = \dfrac{224}{1,1.1000}.4,5 = 0,9(mol)$
$NaOH + HNO_3 \to NaNO_3 + H_2O$
$Ba(OH)_2 + 2HNO_3 \to Ba(NO_3)_2 + 2H_2O$
Theo PTHH :
$0,005a + 0,0005a.2 = 0,9 \Rightarrow a = 150(gam)$
Đúng 2
Bình luận (0)
Câu 2 :
Gọi thể tích dung dịch cần tìm là V(lít)
$n_{H_2SO_4} = 1,1V(mol) ; n_{HCl} = 1,98V(mol)$
$\Rightarrow n_H = 1,1V.2 + 1,98V = 4,18V(mol)$
$n_{NaOH} = 0,19.3 = 0,57(mol) ; n_{Ba(OH)_2} = 0,19.4 = 0,76(mol)$
$\Rightarrow n_{OH} = 0,57 + 0,76.2 = 2,09(mol)$
$H + OH \to H_2O$
$\Rightarrow 4,18V = 2,09 \Rightarrow V = 0,5(lít)$
Đúng 1
Bình luận (0)
Câu 3 :
$n_{ZnO} = \dfrac{48,6}{81} = 0,6(mol) ; n_{HNO_3} = \dfrac{800.6,3\%}{63}= 0,8(mol)$
ZnO + 2HNO3 → Zn(NO3)2 + H2O
0,4.......0,8...............0,4........................(mol)
Sau phản ứng :
$m_{dd} = 0,4.81 +800 = 832,4(gam)$
$C\%_{Zn(NO_3)_2} = \dfrac{0,4.189}{832,4}.100\% = 9,1\%$
Đúng 2
Bình luận (0)
Xem thêm câu trả lời



















