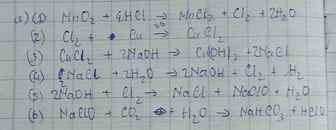Cl2→NaCl→AgCl

Những câu hỏi liên quan
a. NaCl → Cl2 → FeCl3 → NaCl → HCl → CuCl2 → AgCl.b. CaCO3 → CaCl2 → NaCl → NaOH → NaClO → NaCl → Cl2 → FeCl3 → AgClc. KMnO4 → Cl2 → KClO3 → KCl → HCl → CuCl2 → AgCl → Cl2 → clorua vôid. Cl2 → KClO3 → KCl → Cl2 → HCl → FeCl2 → FeCl3 → PbCl2.
Đọc tiếp
a. NaCl → Cl2 → FeCl3 → NaCl → HCl → CuCl2 → AgCl.
b. CaCO3 → CaCl2 → NaCl → NaOH → NaClO → NaCl → Cl2 → FeCl3 → AgCl
c. KMnO4 → Cl2 → KClO3 → KCl → HCl → CuCl2 → AgCl → Cl2 → clorua vôi
d. Cl2 → KClO3 → KCl → Cl2 → HCl → FeCl2 → FeCl3 → PbCl2.
a. NaCl → Cl2 → FeCl3 → NaCl → HCl → CuCl2 → AgCl.
2NaCl+2H2O-đp\mn->2NaOH+H2+Cl2
3Cl2+2Fe-to>2FeCl3
FeCl3+3NaOH->3NaCl+Fe(OH)3
2NaCl+H2SO4-to>Na2SO4+2HCl
2HCl+CuO->CuCl2+H2O
CuCl2+2AgNO3->2AgCl+Cu(NO3)2
Đúng 3
Bình luận (0)
b. CaCO3 → CaCl2 → NaCl → NaOH → NaClO → NaCl → Cl2 → FeCl3 → AgCl
CaCO3+2HCl->CaCl2+H2O+CO2
CaCl2+Na2CO3->2NaCl+CaCO3
2NaCl+2H2o-đp->2NaOH+Cl2+H2
2NaOH+Cl2-to>NaCl+NaClO+H2O
2NaClO-to->2NaCl+O2
2NaCl+2H2O-đp\mn->2NaOH+H2+Cl2
3Cl2+2Fe-to>2FeCl3
FeCl3+3AgNO3->3AgCl+Fe(NO3)3
Đúng 1
Bình luận (0)
Hoàn thành các chuỗi biến hóa sau :
HCl Cl2 FeCl3 NaCl AgCl
KMnO4 Cl2 HCl FeCl2 FeCl3 AgCl Cl2 Br2
a)
\(4HCl+MnO_2\rightarrow MnCl_2+Cl_2+2H_2O\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
\(FeCl_3+3NaOH\rightarrow3NaCl+Fe\left(OH\right)_3\)
\(NaCl+AgNO_3\rightarrow NaNO_3+AgCl\)
b)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl2+5Cl_2+8H_2O\)
\(Cl_2+H_2\rightarrow2HCl\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2FeCl_2+Cl_2\rightarrow2FeCl_3\)
\(FeCl_3+3AgNO_3\rightarrow Fe\left(NO_3\right)_3+3AgCl\)
\(2AgCl\rightarrow2Ag+Cl_2\)
\(Cl_2+2NaBr\rightarrow2NaCl+Br_2\)
H2----> HCl----> Cl2----> NaClO------> Na2SO4------> NaCl---->AgCl
\(H_2 + Cl_2 \xrightarrow{as} 2HCl\\ MnO_2 + 4HCl \to MnCl_2 + Cl_2 + 2H_2O\\ Cl_2 + 2NaOH \to NaCl + NaClO + H_2O\\ 2NaClO+ H_2SO_4 \to Na_2SO_4 + 2HClO\\ Na_2SO_4 + BaCl_2 \to BaSO_4 + 2NaCl\\ NaCl + AgNO_3 \to AgCl + NaNO_3\)
Đúng 1
Bình luận (0)
KClO3 ----->Cl2-------> FeCl3-------> NaCl----> HCl--->CuCl2---->AgCl
Br2 + 2KClO3 \(\xrightarrow[]{t^o}\) 2KBrO3 + Cl2
Fe + Cl2 \(\xrightarrow[]{t^o}\) FeCl3
3NaOH + FeCl3 \(\rightarrow\) 3NaCl + Fe(OH)3
NaCl + H2SO4 \(\rightarrow\) NaHSO4 + HCl
CuO + 2HCl \(\rightarrow\) CuCl2 + H2O
2AgNO3 + CuCl2\(\xrightarrow[]{t^o}\) 2AgCl + Cu(NO3)2
Đúng 2
Bình luận (0)
Thực hiện các chuỗi sau
MnO2 -> Cl2 -> HCl -> NaCl -> Cl2 -> AlCl3 -> Al(OH)3 -> AlCl3 -> AgCl
\(MnO_2+4HCl_{đặc,nóng}\rightarrow MnCl_2+Cl_2+2H_2O\\ H_2+Cl_2\rightarrow\left(t^o\right)2HCl\\ NaOH+HCl\rightarrow NaCl+H_2O\\ 2NaCl\rightarrow\left(đpnc\right)2Na+Cl_2\\ 2Al+3Cl_2\rightarrow\left(t^o\right)2AlCl_3\\ AlCl_3+3KOH\rightarrow Al\left(OH\right)_3\downarrow+3KCl\\ Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2o\\ AlCl_3+3AgNO_3\rightarrow Al\left(NO_3\right)_3+3AgCl\downarrow\)
Đúng 3
Bình luận (0)
\(MnO_2\underrightarrow{1}Cl\underrightarrow{2}HCl\underrightarrow{3}NaCl\underrightarrow{4}Cl_2\underrightarrow{5}AlCl_3\underrightarrow{6}Al\left(OH\right)_3\underrightarrow{7}AlCl_3\underrightarrow{8}AgCl\)
(1) \(MnO_2+4HCl_{đặc}\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\)
(2) \(H_2+Cl_2\xrightarrow[ánh.sáng]{t^o}2HCl\)
(3) \(NaOH+HCl\rightarrow NaCl+H_2O\)
(4) \(2NaCl+2H_2O\xrightarrow[có.màng.ngăn]{điện.phân}2NaOH+H_2+Cl_2\)
(5) \(2Al+3Cl_2\underrightarrow{t^o}2AlCl_3\)
(6) \(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\)
(7) \(Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2O\)
(8) \(AlCl_3+3AgNO_3\rightarrow Al\left(NO_3\right)_3+3AgCl\)
Chúc bạn học tốt
Đúng 1
Bình luận (0)
Viết các phương trình phản ứng xảy ra cho các sơ đồ sau:1. HCl - Cl2 - FeCl3 - NaCl - HCl - CuCl2 - AgCl2. KMnO4 -Cl2-HCl -FeCl3 - AgCl - Cl2-Br2-I23. KMnO4 → Cl2 → HCl →FeCl2 → AgCl → Ag4. HCl → Cl2→ FeCl3 → Fe(OH)3 → Fe2(SO4)3
Đọc tiếp
Viết các phương trình phản ứng xảy ra cho các sơ đồ sau:
1. HCl -> Cl2 -> FeCl3 -> NaCl -> HCl -> CuCl2 -> AgCl
2. KMnO4 ->Cl2->HCl ->FeCl3 -> AgCl -> Cl2->Br2->I2
3. KMnO4 → Cl2 → HCl →FeCl2 → AgCl → Ag
4. HCl → Cl2→ FeCl3 → Fe(OH)3 → Fe2(SO4)3
1,
\(4HCl+MnO_2\rightarrow MnCl_2+2H_2O+Cl_2\\ 2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3\\ FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\\ 2NaCl+H_2SO_4\rightarrow Na_2SO_4+2HCl\uparrow\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ CuCl_2+2AgNO_3\rightarrow2AgCl\downarrow+Cu\left(NO_3\right)_2\)
2,
\(2KMnO_4+16HCl\rightarrow2KCl+8H_2O+5Cl_2+2MnCl_2\\ Cl_2+H_2\underrightarrow{as}2HCl\\ 6HCl+Fe_2O_3\rightarrow2FeCl_3+3H_2O\\ FeCl_3+3AgNO_3\rightarrow3AgCl\downarrow+Fe\left(NO_3\right)_3\\ 2AgCl\underrightarrow{as}2Ag+Cl_2\\ Cl_2+2NaBr\rightarrow2NaCl+Br_2\\ Br_2+2NaI\rightarrow2NaBr+I_2\)
3,
2 pthh đầu giống ở 2
\(Fe+2HCl\rightarrow FeCl_2+H_2\\ FeCl_2+2AgNO_3\rightarrow2AgCl\downarrow+Fe\left(NO_3\right)_2\\ 2AgCl\underrightarrow{as}2Ag+Cl_2\)
4, 2 pthh đầu gióng ở 1
\(FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\\ 2Fe\left(OH\right)_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+6H_2O\)
Đúng 3
Bình luận (0)
1) hoàn thành sơ đồ
(1) KMnO4 --> O2
(2) O2 --> Fe2O3
(3) Fe2O3 --> FeCl3
(4) FeCl3 --> FeOH3
(5) KMnO4 --> Cl2
(6) Cl2 --> Nacl
(7) Nacl --> Agcl
(8) Agcl --> Cl2
(1) 2KMnO4 =nhiệt độ=> K2MnO4 + MnO2 + O2
(2) O2 + 4Fe(OH)2 =nhiệt độ=> 2Fe2O3 + 4H2O
(3) Fe2O3 + 6HCl => 2FeCl3 + 3H2O
(4) FeCl3 + 3NaOH => Fe(OH)3 + 3NaCl
(5) 2KMnO4 + 16HCl => 2KCl + 2MnCl2 + 5Cl2 + 8H2O
(6) Cl2 + 2Na =nhiệt độ=> 2NaCl
(7) NaCl + AgNO3 => AgCl + NaNO3
(8) 2AgCl =nhiệt độ=> 2Ag + Cl2
Đúng 0
Bình luận (0)
Hoàn thành phương trình phản ứng theo chuỗi biên hóa sau (ghi rõ đk nếu có)
a/ MnO2 Cl2→ CuCl2→ NaCl →NAOH → NaClO -→ HC1O
b/ KMNO4→ Cl2→ HCl → KCI → KOH → KCI → Cl2→ FeCl3→ Fe(N03)3
c/ Cl2→ NaCl → AgCl → Cl2→ NaClO –→ Cl2→ CaOCl2
d/ H2 HCl - FeCl2 FeCl3→NaCl → HCI → CaCl2→ CACO3
$a) MnO_2 + 4HCl \xrightarrow{t^o} MnCl_2 + Cl_2 + 2H_2O$
$Cl_2 + Cu \xrightarrow{t^o} CuCl_2$
$CuCl_2 + 2NaOH \to Cu(OH)_2 + 2NaCl$
$2NaCl + 2H_2O \xrightarrow{đpdd, cmn} 2NaOH + H_2 + Cl_2$
$2NaOH + Cl_2 \to NaCl + NaClO + H_2O$
$NaClO + HCl \to NaCl + HClO$
b)
$2KMnO_4 + 16HCl \to 2KCl + 2MnCl_2 + 5Cl_2 + 8H_2O$
$Cl_2 + H_2 \xrightarrow{ánh\ sáng} 2HCl$
$HCl + KOH \to KCl + H_2O$
$2KCl + 2H_2O \xrightarrow{đpdd, cmn} 2KOH + H_2 + Cl_2$
$3Cl_2 + 2Fe \xrightarrow{t^o} 2FeCl_3$
$FeCl_3 + 3AgNO_3 \to Fe(NO_3)_3 + 3AgCl$
Đúng 6
Bình luận (4)
1,KI-I2-HI-HCL-KCL-CL2-HCLO-O2-CL2-BR2-I2
2,KMNO4-CL2-HCL-FECL2-AGCL-AG
3, KMnO4- Cl2- HCl- FeCl3- AgCl- Cl2- Br2- I2- ZnI2- Zn(OH)2
4,KCL-CL2-KCLO- KCLO3-KCLO4-KCL-KNO3
5,CL2-KCLO3-KCL-CL2-CA(CLO)2-CL2
6, KMNO4-CL2-KCLO3-KCL-CL2-HCL-FECL2-FECL3-FE(OH)2
7,CACL2- NACL-HCL-CL2-CAOCL2-CACO3-CACL2--NACL-NACL
8, HCL- CL2- FECL3-FE(OH)3-FES2(SO4)3
9,HCL-CL2-NACL-HCL-CUCL2-.AGCL-AG
10,MNO2-CL2-KCLO3-KCL-HCL-CL2-CHORUA VÔI
Đọc tiếp
1,KI->I2->HI->HCL->KCL->CL2->HCLO->O2->CL2->BR2->I2
2,KMNO4->CL2->HCL->FECL2->AGCL->AG
3, KMnO4-> Cl2-> HCl-> FeCl3-> AgCl-> Cl2-> Br2-> I2-> ZnI2-> Zn(OH)2
4,KCL->CL2->KCLO-> KCLO3->KCLO4->KCL->KNO3
5,CL2->KCLO3->KCL->CL2->CA(CLO)2->CL2
6, KMNO4->CL2->KCLO3->KCL->CL2->HCL->FECL2->FECL3->FE(OH)2
7,CACL2-> NACL->HCL->CL2->CAOCL2->CACO3->CACL2-->NACL->NACL
8, HCL-> CL2-> FECL3->FE(OH)3->FES2(SO4)3
9,HCL->CL2->NACL->HCL->CUCL2-.AGCL->AG
10,MNO2->CL2->KCLO3->KCL->HCL->CL2-CHORUA VÔI
2) 2KMnO4 + 16HCl = 2KCl + 2MnCl2 + 5Cl2 + 8H2O
Cl2 + H2 = 2HCl ( điều kiện ánh sáng )
2HCl + Fe = FeCl2 + H2
FeCl2 + 2AgNO3 = 2AgCl + Fe(NO3)2
2AgCl = 2Ag + Cl2
Đúng 0
Bình luận (0)
10. MnO2 + 4HCl = MnO2 + Cl2 + H2O (nhiệt độ)
3Cl2 + 6KOH(đặc) = 5KCl + KClO3 + 3H2O(nhiệt độ)
KClO3 = KCl + 3O2(nhiệt độ)
KCl(rắn) + H2SO4 = 2HCl + K2SO4 (nhiệt độ)
4HCl + MnO2 = MnO2 + Cl2 + H2O(nhiệt độ)
Cl2 + Ca(OH)2 = CaOCl2 + H2O
Đúng 0
Bình luận (0)