mn giúp mình gấp ạ

Những câu hỏi liên quan
mn giúp mình ạ , mình cần gấp , mình cảm ơn ạ
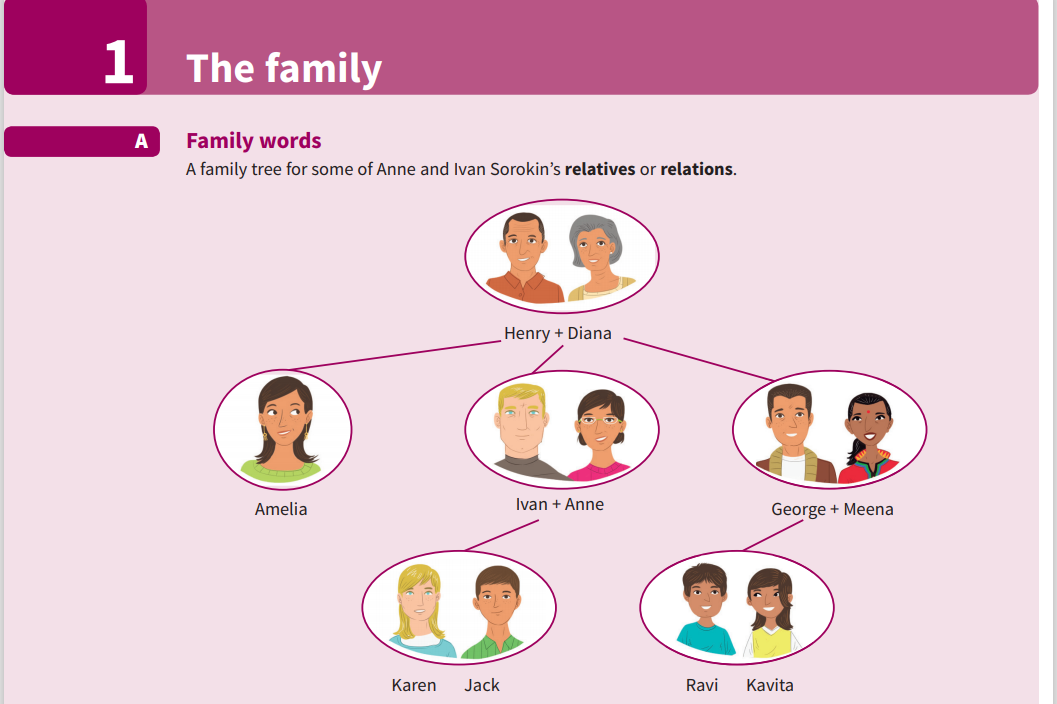

Mn ơi giúp mình với ạ mình cần gấp ạ
1 his illness, he cannot come
2 her busyness, she couldn't help us
3 his illness, he tries to go to school on time
4 the bad weather, we tried to finish the work on the road
5 the bad weather, we got to the station late
6 the old house, she liked it
7 not wearing any shoes, Carol ran outside to see what was happening
8 being afraid of flying, Fiona had to get on the plane
Đúng 2
Bình luận (0)
mn ơi giúp mình với ạ, mình cần gấp,mình cảm ơn mn
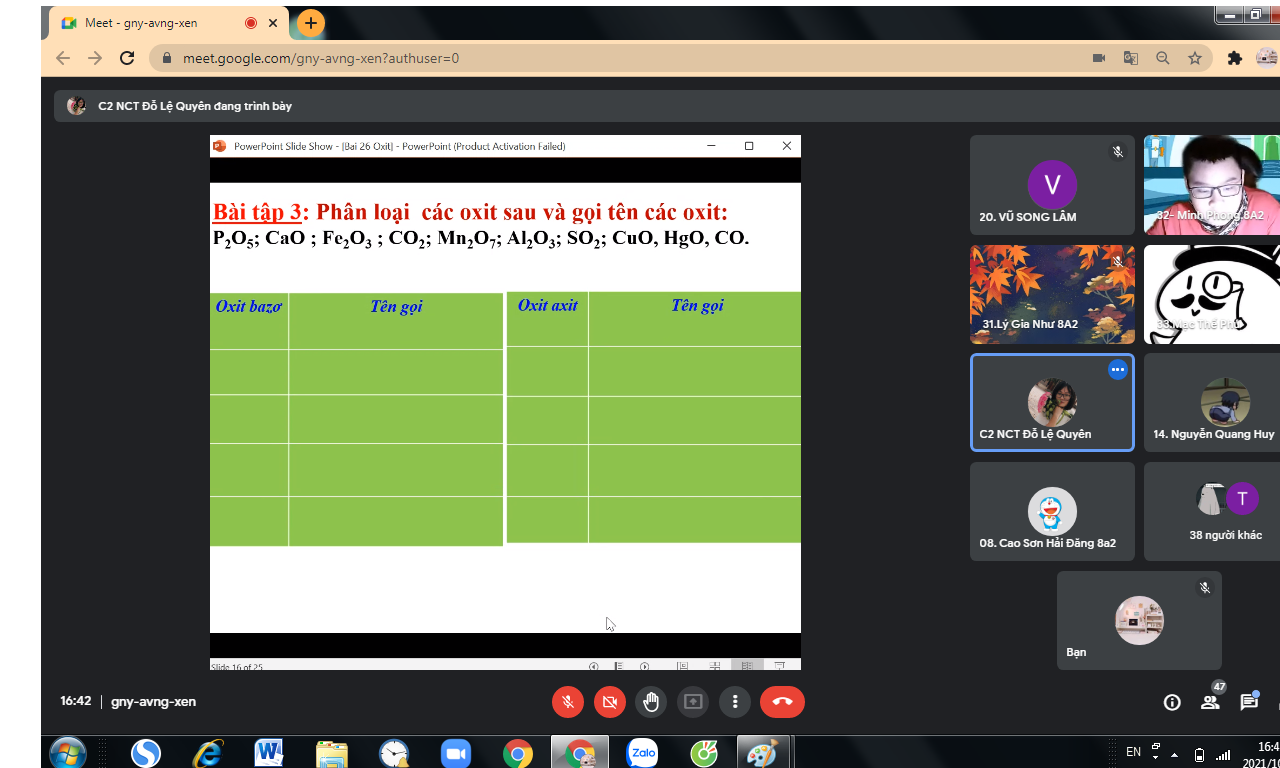
Giúp mình với mn ạ. Mình đang cần gấp ạ 🥺
\(x^4-8x=x\left(x^3-8\right)=x\left(x-2\right)\left(x^2+2x+4\right)\)
\(x^2-y^2-6x+9=\left(x^2-6x+9\right)-y^2=\left(x-3\right)^2-y^2=\left(x+y-3\right)\left(x-y-3\right)\)
Đúng 1
Bình luận (1)
Giúp mình với ạ, mình cần gấp. Cảm ơn mn ạ
Ai giải giúp mình với ạ ! Cần gấp mong mn giúp ạ
Giúp mình với mình đang cần gấp mong mn làm giúp mình ạ
1 How long do you live in HN
2 How often did you go to the movies
3 What did Mrs Robinson buy
4 When was your father in Nghe An
5 How do they go to school
6 Why didn't she go to class
7 Who left home at 7o'clock yesterday
8 What subject does he study in the high school
9 What is your favorite hobby
10 How far is it
Đúng 1
Bình luận (1)
Mn giúp mình với ạ. Mình cần gấp lắm ạ mai mình phải nộp ròi
Giúp mình với ạ , mình đang cân gấp , xin cảm ơn mn ạ
Bài 4 :
\(n_{H2}=\dfrac{V_{H2}}{22,4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2|\)
2 3 1 3
0,1 0,15 0,05 0,15
a) \(n_{Al}=\dfrac{0,15.2}{3}=0,1\left(mol\right)\)
⇒ \(m_{Al}=n_{Al}.M_{Al}\)
= 0,1 . 27
= 2,7 (g)
\(m_{Cu}=10-2,7=7,3\left(g\right)\)
0/0Al = \(\dfrac{m_{Al}.100}{m_{hh}}=\dfrac{2,7.100}{10}=27\)0/0
0/0Cu = \(\dfrac{m_{Cu}.100}{m_{hh}}=\dfrac{7,3.100}{10}=13\)0/0
b) \(n_{Al2\left(SO4\right)3}=\dfrac{0,15.1}{3}=0,05\left(mol\right)\)
⇒ \(m_{Al2\left(SO4\right)3}=n_{Al2\left(SO4\right)3.}M_{Al2\left(SO4\right)3}\)
= 0,05 . 342
= 17,1 (g)
\(n_{H2SO4}=\dfrac{0,1.3}{2}=0,15\left(mol\right)\)
⇒ \(m_{H2SO4}=n_{H2SO4}.M_{H2SO4}\)
= 0,15 .98
= 14,7 (g)
\(C_{H2SO4}=\dfrac{m_{ct}.100}{m_{dd}}\Rightarrow m_{dd}=\dfrac{m_{ct}.100}{C}=\)\(\dfrac{14,7.100}{15}=98\left(g\right)\)
mdung dịch sau phản ứng = (mAl + mCu) + mH2SO4 - mH2
= 10 + 98 - (0,15 . 2)
=107,7 (g)
\(C_{Al2\left(SO4\right)3}=\dfrac{m_{ct}.100}{m_{dd}}=\dfrac{17,1.100}{107,7}=15,88\)0/0
Chúc bạn học tốt
Đúng 1
Bình luận (1)
Mn giúp đỡ mình với ạ
Mình đang cần gấp lắm ạ🙏
























