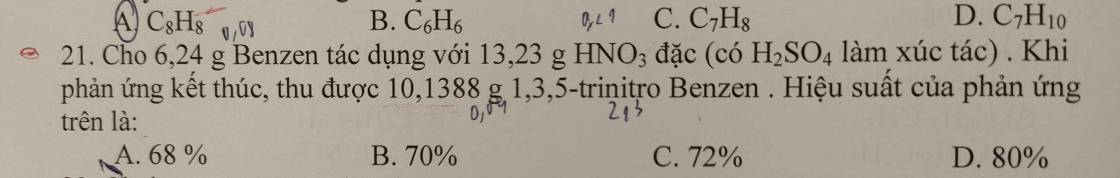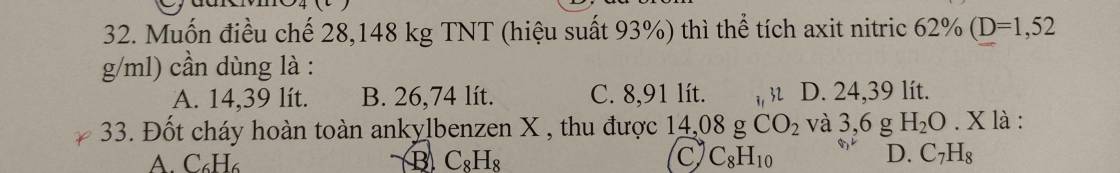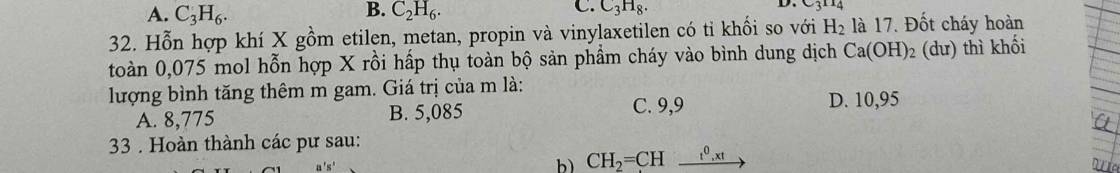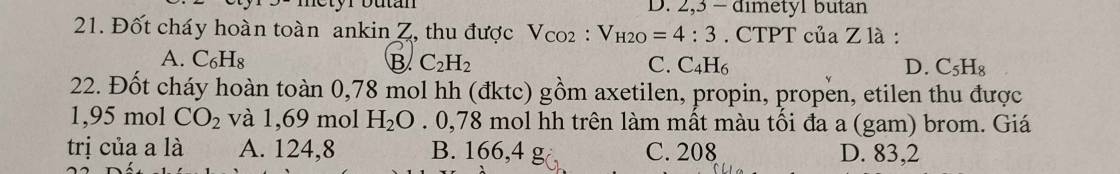Câu 21:
\(n_{C_6H_6}=\dfrac{6,24}{78}=0,08\left(mol\right)\)
\(n_{HNO_3}=\dfrac{13,23}{63}=0,21\left(mol\right)\)
PT: \(C_6H_6+3HNO_3\underrightarrow{^{t^o,xt}}C_6H_3\left(NO_2\right)_3+3H_2O\)
Xét tỉ lệ: \(\dfrac{0,08}{1}>\dfrac{0,21}{3}\), ta được C6H6 dư.
Theo PT: \(n_{C_6H_3\left(NO_2\right)_3\left(LT\right)}=\dfrac{1}{3}n_{HNO_3}=0,07\left(mol\right)\)
\(\Rightarrow m_{C_6H_3\left(NO_2\right)_3\left(LT\right)}=0,07.213=14,91\left(g\right)\)
\(\Rightarrow H\%=\dfrac{10,1388}{14,91}.100\%=68\%\)
→ Đáp án: A
Câu 32:
Ta có: \(n_{C_7H_5\left(NO_2\right)_3\left(TT\right)}=\dfrac{28,148}{227}=0,124\left(kmol\right)\)
\(\Rightarrow n_{C_7H_5\left(NO_2\right)_3\left(TT\right)}=\dfrac{0,124}{93\%}=\dfrac{2}{15}\left(kmol\right)\)
BTNT N, có: \(n_{HNO_3}=3n_{C_7H_5\left(NO_2\right)_3}=0,4\left(kmol\right)\)
\(\Rightarrow m_{HNO_3}=0,4.63=25,2\left(kg\right)\Rightarrow m_{ddHNO_3}=\dfrac{25,2}{62\%}=\dfrac{1260}{31}\left(kg\right)\)
\(\Rightarrow V_{ddHNO_3}=\dfrac{\dfrac{1260}{31}}{1,52}\approx26,74\left(l\right)\)
Đáp án: B