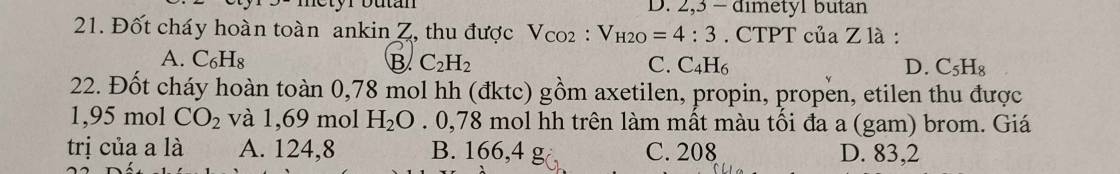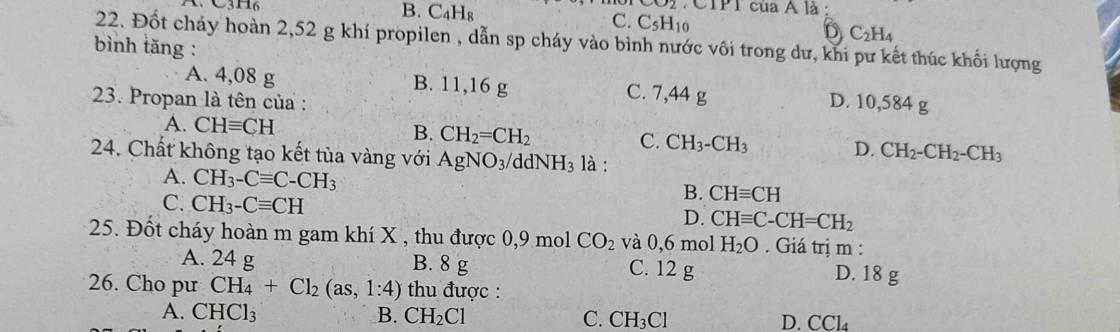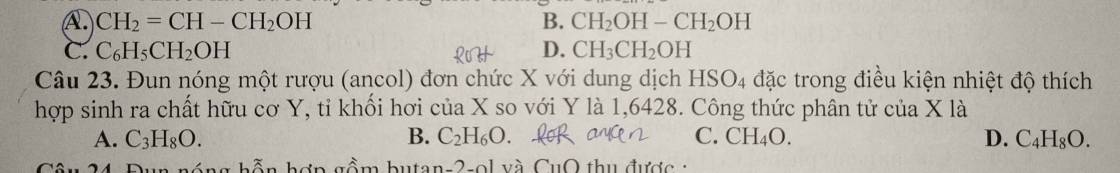Câu 21:
\(C_nH_{2n-2}+AgNO_3+NH_3\rightarrow C_nH_{2n-3}Ag+NH_4NO_3\\ n_X=\dfrac{14-5,44}{108-1}=0,08\left(mol\right)=n_{kết.tủa}\\ M_{C_nH_{2n-3}Ag}=\dfrac{14}{0,08}=175\left(\dfrac{g}{mol}\right)=14n+107\\ \Leftrightarrow n=5\\ \Rightarrow CTPT.X:C_5H_8\\ CTCT:CH\equiv C-CH_2-CH_2-CH_3\\ CH_3-C\equiv C-CH_2-CH_3\\ CH\equiv C-CH\left(CH_3\right)-CH_3\)
Chọn C
Câu 22:
\(n_{C_2H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ n_{Br_2}=2.n_{C_2H_2}=2.0,1=0,2\left(mol\right)\\ m_{Br_2}=0,2.160=32\left(g\right)\\ Chọn.A\)
Câu 23:
\( PTHH:C_2H_2+2Br_2\rightarrow C_2H_2Br_4\left(1\right)\\ C_2H_4+Br_2\rightarrow C_2H_4Br_2\left(2\right)\\ C_2H_2+2AgNO_3+2NH_3\rightarrow Ag_2C_2+2NH_4NO_3\\ n_{Ag_2C_2}=\dfrac{18}{240}=0,075=n_{C_2H_2}\\ n_{Br_2\left(tổng\right)}=\dfrac{40}{160}=0,25\left(mol\right)\\ n_{Br_2\left(1\right)}=2.n_{C_2H_2}=2.0,075=0,15\left(mol\right)\\ n_{Br_2\left(2\right)}=0,25-0,15=0,1\left(mol\right)\\ V=V_{C_2H_2\left(đktc\right)}+V_{C_2H_4\left(đktc\right)}=0,075.22,4+0,1.22,4=3,92\left(lít\right)\\ Chọn.A\)