\(Câu1:\\ n_{CO_2}=\dfrac{m}{M}=\dfrac{11}{44}=0,25\left(mol\right)\\ Số.phân.tử=0,25.6.10^{23}=15.10^{22}\left(phân.tử\right)\)
\(n_{H_2}=\dfrac{9.10^{23}}{6.10^{23}}=1,5\left(mol\right)\\ V_{H_2}=1,5.22,4=33,6\left(l\right)\)
\(Câu2:\)
\(n_{O_2}=\dfrac{V}{22,4}=\dfrac{67,2}{22,4}=3\left(mol\right)\\ Số.phân.tử=3.6.10^{23}=18.10^{23}\left(phân.tử\right)\\ m_{O_2}=n.M=3.32=96\left(g\right)\)








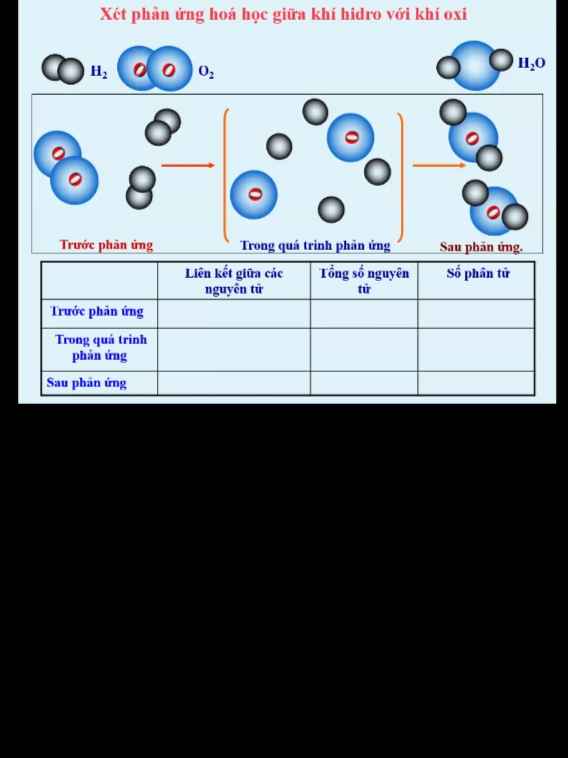










 GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP
GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP