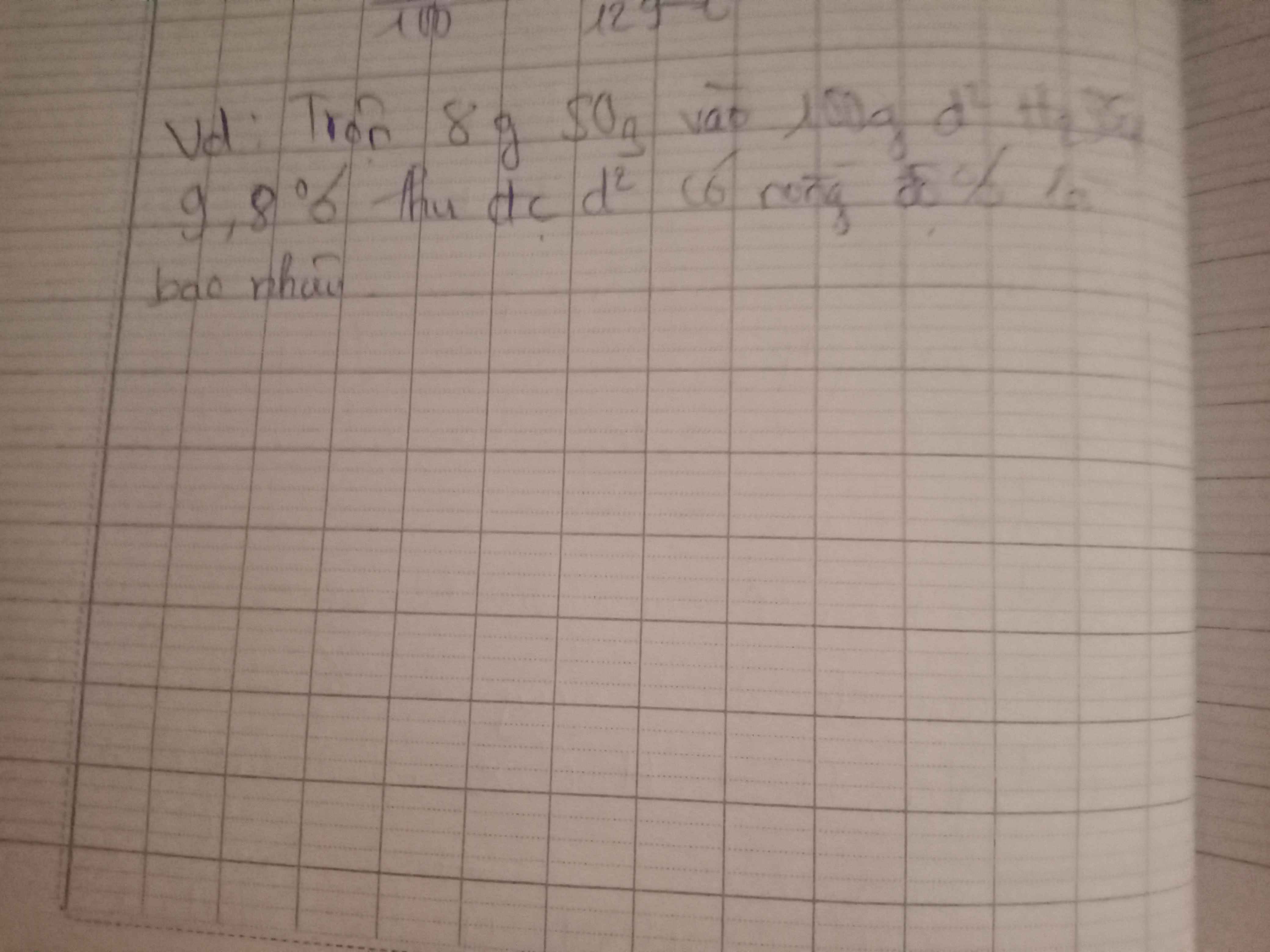
\(m_{NaOH}=a\left(g\right),V_{dd_{NaOH}}=b\left(l\right)\)
\(n_{NaOH}=\dfrac{a}{40}\left(mol\right)\)
\(n_{NaOH}=0.5b\left(mol\right)\)
\(n_{NaOH\left(2M\right)}=2.5\cdot2=5\left(mol\right)\)
\(\Rightarrow\dfrac{a}{40}+0.5b=5\left(1\right)\)
\(m_{dd_{NaOH}}=2500\cdot1.06=2650\left(g\right)\)
\(\Rightarrow a+1000b\cdot1.06=2650\left(2\right)\)
\(\left(1\right),\left(2\right):\)
Số lẻ quá em ơi :<
\(\)
mNaOH=a(g),VddNaOH=b(l)mNaOH=a(g),VddNaOH=b(l)
⇒a40+0.5b=5(1)⇒a40+0.5b=5(1)
mddNaOH=2500⋅1.06=2650(g)mddNaOH=2500⋅1.06=2650(g)
⇒a+1000b⋅1.06=2650(2)⇒a+1000b⋅1.06=2650(2)
(1),(2):