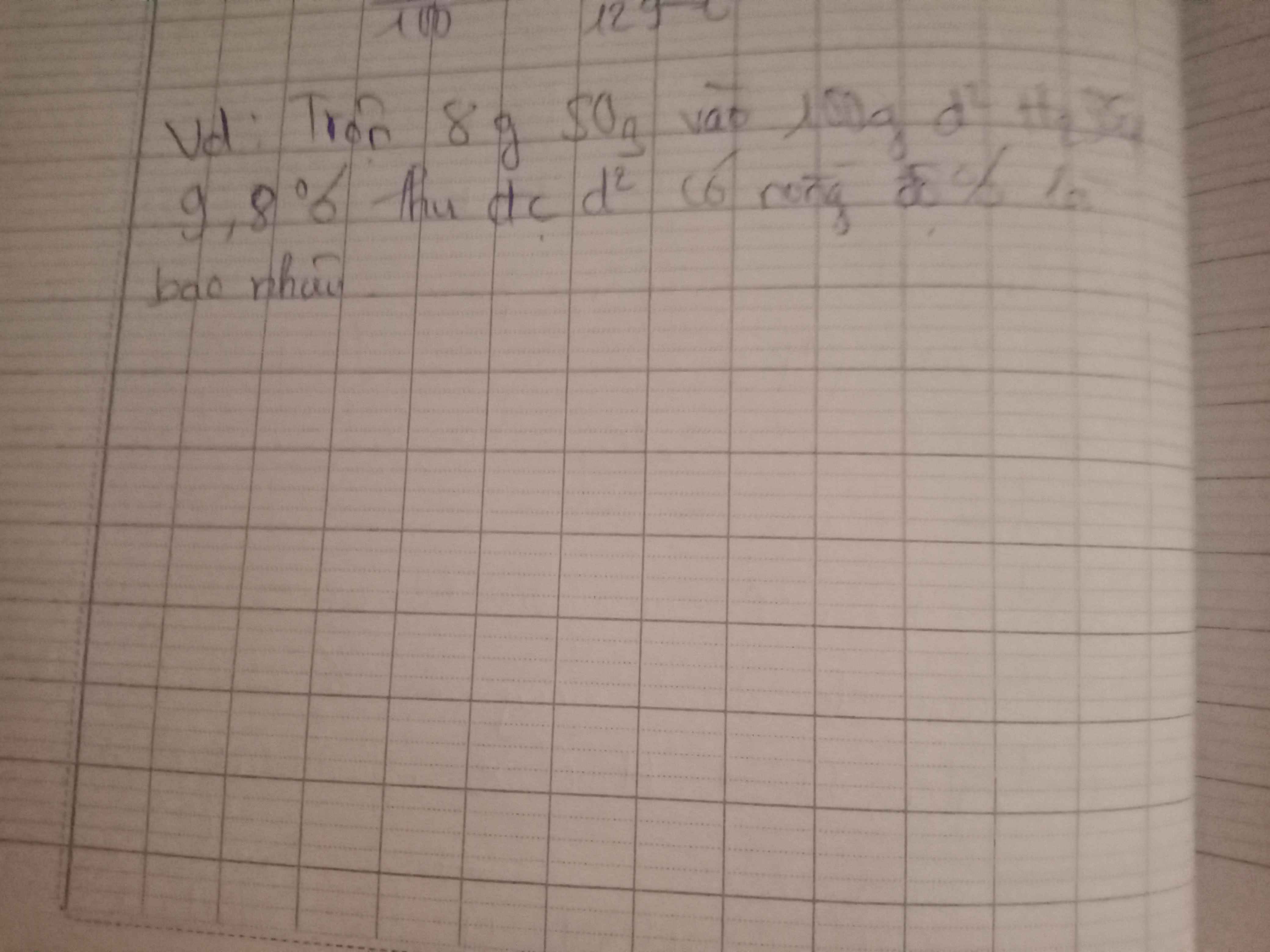
Câu 6:
Gọi kim loại đó là \(R\)
\(\rightarrow Oxit:R_2O_3\)
Giả sử dd \(H_2SO_4\) phản ứng \(a\left(mol\right)\)
\(PTHH:R_2O_3+3H_2SO_4\rightarrow R_2\left(SO_4\right)_3+3H_2O\)
\(\left(mol\right)\) \(\dfrac{a}{3}\) \(a\) \(\dfrac{a}{3}\)
\(m_{ddH_2SO_4}=\dfrac{98a.100}{10}=980a\left(g\right)\)
\(C\%_{ddspu}=12,9\left(\%\right)\Leftrightarrow\dfrac{\left(2R+288\right).\dfrac{a}{3}}{\left(2R+48\right).\dfrac{a}{3}+980a}.100=12,9\\ \Leftrightarrow\dfrac{\dfrac{\left(2R+288\right)}{3}}{\dfrac{\left(2R+48\right)}{3}+980}.100=12,9\\ \Leftrightarrow R=56\left(Fe\right)\\ \rightarrow Oxit:Fe_2O_3\)
Câu 7:
\(a.n_{NaOH}=\dfrac{60.10\%}{40}=0,15\left(mol\right)\)
Đặt \(C\%_{HCl}=a\left(\%\right)\Rightarrow n_{HCl}=\dfrac{40a}{100.36,5}=\dfrac{4a}{365}\left(mol\right)\)
\(C\%_{NaCl}=5,85\%\Leftrightarrow\dfrac{m_{NaCl}}{60+40}.100=5,85\Leftrightarrow m_{NaCl}=5,85\left(g\right)\Leftrightarrow n_{NaCl}=0,1\left(mol\right)\)
\(PTHH:NaOH+HCl\rightarrow NaCl+H_2O\)
(mol) 0,1 0,1 0,1
Lúc này ta có: \(n_{HCl}=\dfrac{4a}{365}=0,1\Leftrightarrow a=9,125\left(\%\right)\)
Câu b làm tương tự!!!