Bài 1:
\(a,Magnesium+Oxygen\xrightarrow{t^o}Magnesium Oxide\\ b,m_{Mg}+m_{O_2}=m_{MgO}\\ c,m_{O_2}=m_{MgO}-m_{Mg}=15-9=6(g)\)
Bài 2:
\(a,Sulfur+Oxygen\xrightarrow{t^o}\text {Sulfur dioxide}\\ b,m_{S}+m_{O_2}=m_{SO_2}\\ c,m_{O_2}=m_{SO_2}-m_{S}=6,4-3,2=3,2(g)\)
Bài 3:
\(a,zinc+\text{hydrochloric acid}\to \text {zinc chloride}+hydrogen\\ b,m_{Zn}+m_{HCl}=m_{ZnCl_2}+m_{H_2}\\ c,m_{HCl}=m_{ZnCl_2}+m_{H_2}-m_{Zn}=13,6+0,2-6,5=7,3(g)\)







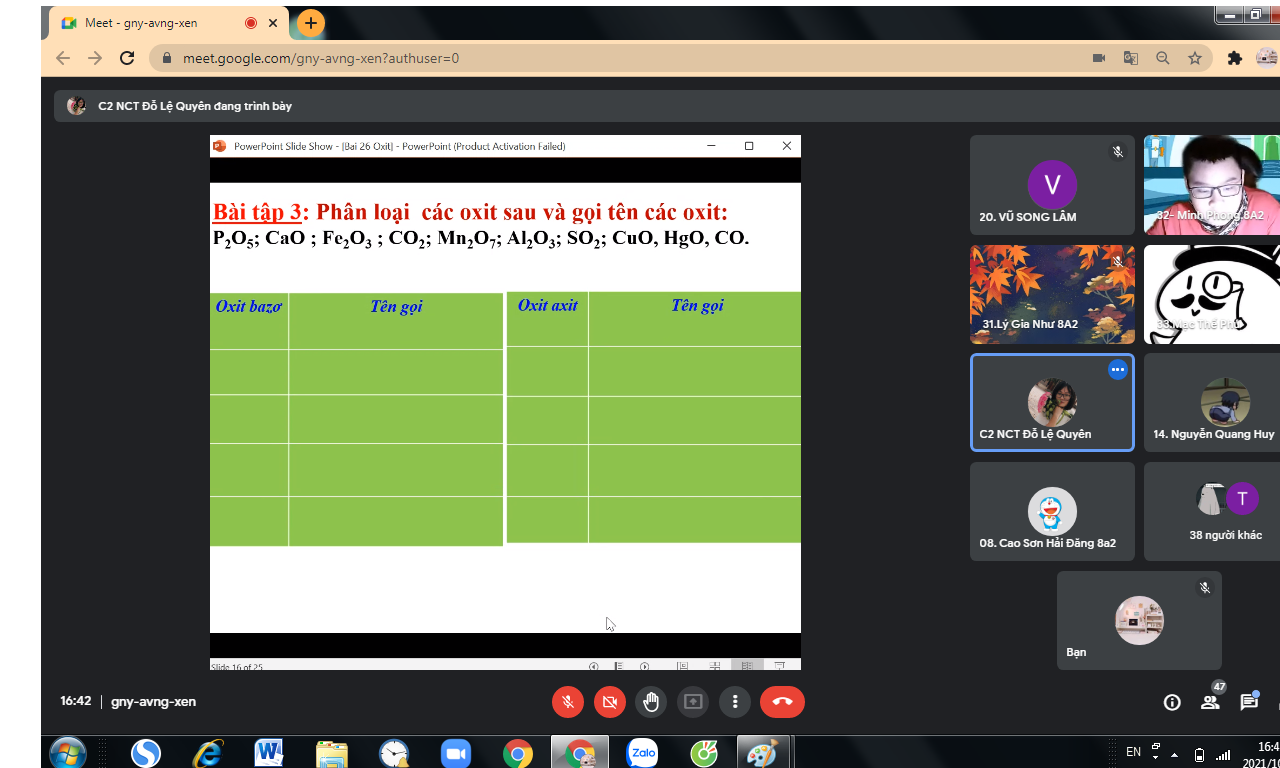








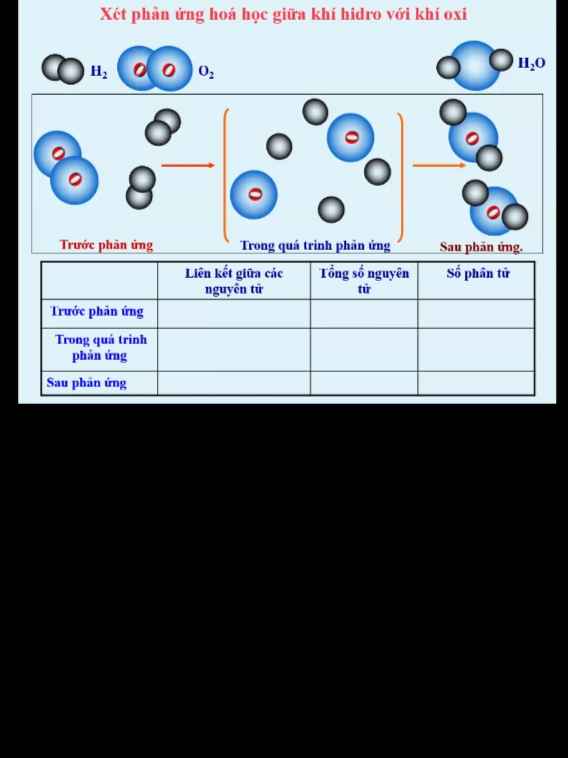

 giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha
giúp mình với mình chỉ cần 2 câu 8 với câu 9 thôi nha 



