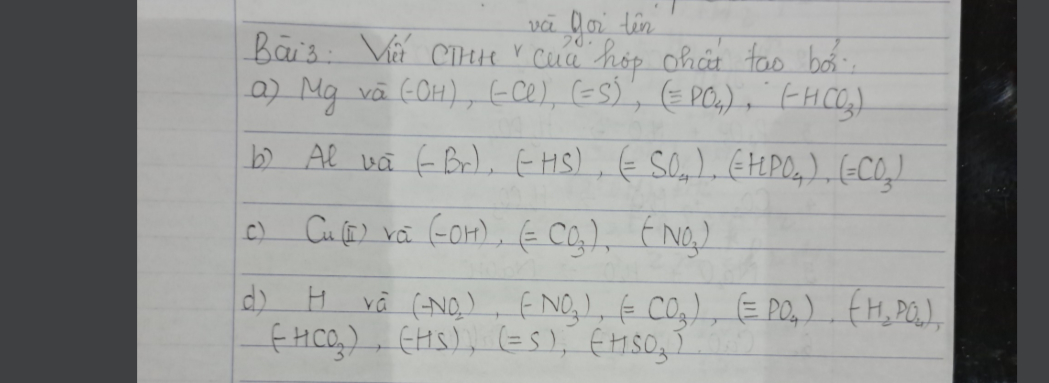\(13.A\\ 24.kí.hiệu?\\ 25.C\\ 26.D\\ 27.C\\ 29.D\\ 30.B\\ 31.B\\ 32.V=1,5.24,79=37,185l\\ \Rightarrow D\\ 33.D\\ 34.n_{O_2}=\dfrac{64}{32}=2mol\\ V_{O_2}=2.24,79=49,58l\\ \Rightarrow B\\ 35.d_{SO_2/Cl_2}=\dfrac{61}{71}\approx0,9\\ \Rightarrow C\\ 36.m_{H_2SO_4}=\dfrac{200.12}{100}=24g\\ \Rightarrow C\\ 36.A\\ 39.n_{HCl}=0,2.1=0,2mol\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{Zn}=n_{H_2}=0,2:2=0,1mol\\ m=m_{Zn}=0,1.65=6,5\\ V=V_{H_2}=0,1.22,4=2,24l\\ \Rightarrow A\)
Đúng 2
Bình luận (1)
Các câu hỏi tương tự
 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
giúp tôi với tối nay 9:00 phải nộp rồi !
cảm ơn nhiều!!!!!giúp tôi các câu còn lại trừ câu 1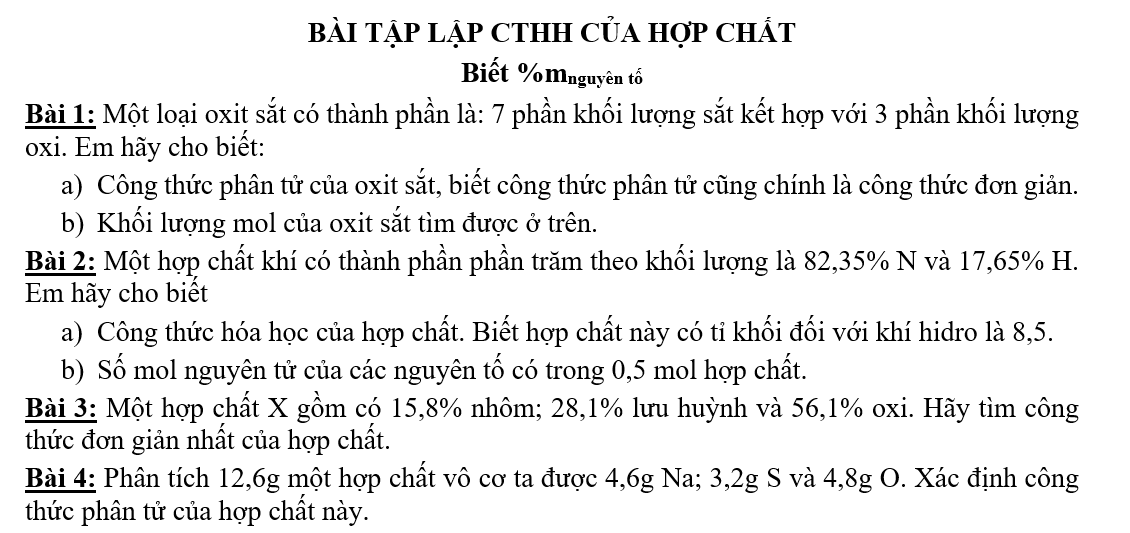
hãy kẻ tên 20 đồ vật đc lm từ 1 chất
GIÚP MIK NHA .CẢM ƠN MN
giúp tôi câu 2 với ! cảm ơn nhiều lắm !!!!
Mn giúp với, cảm ơn nha
Mn giúp mình với, cảm ơn nha
giúp mk nữa nha mn!!
Tính số nguyên tử Na trong 4,6 gam, số phân tử CO2 trong 3,36 lít khí CO2 ( đktc )
Cảm ơn mn nhìu nha
giúp tôi với tôi cần gấp lắm !!
cảm ơn nhiều!!!
tôi chỉ cần các bài còn lại trừ bài 1 thôi!cảm ơn!!!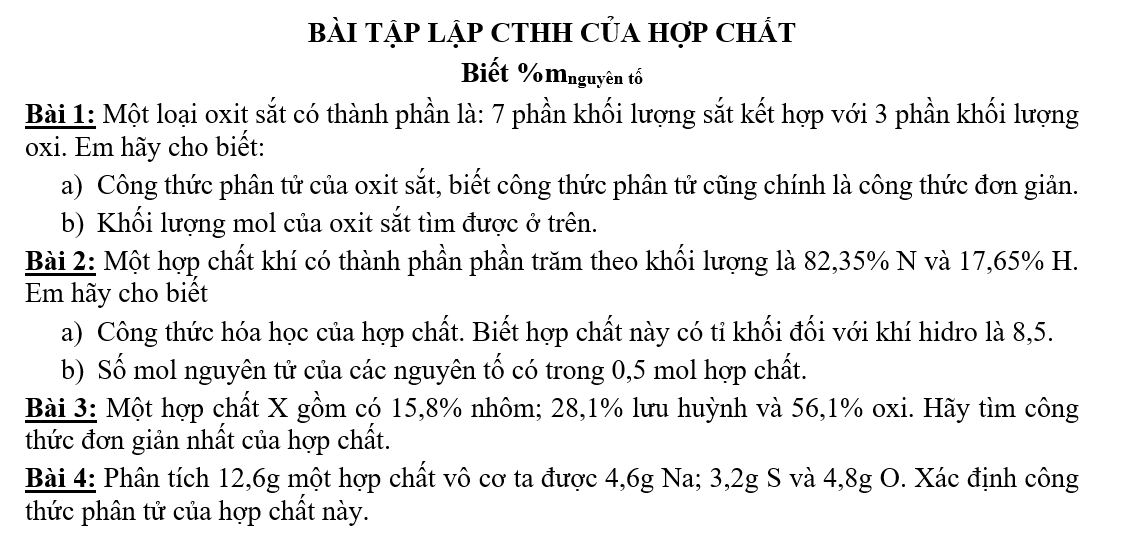
giúp tôi với,tôi cần gấp :(
cảm ơn nhiều !